A randomized controlled trial-based algorithm for insulin-pump therapy in hyperglycemic patients early after kidney transplantation
- PMID: 29518094
- PMCID: PMC5843249
- DOI: 10.1371/journal.pone.0193569
A randomized controlled trial-based algorithm for insulin-pump therapy in hyperglycemic patients early after kidney transplantation
Abstract
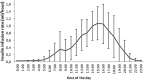
Treating hyperglycemia in previously non-diabetic individuals with exogenous insulin immediately after kidney transplantation reduced the odds of developing Posttransplantation Diabetes Mellitus (PTDM) in our previous proof-of-concept clinical trial. We hypothesized that insulin-pump therapy with maximal insulin dosage during the afternoon would improve glycemic control compared to basal insulin and standard-of-care. In a multi-center, randomized, controlled trial testing insulin isophane for PTDM prevention, we added a third study arm applying continuous subcutaneous insulin lispro infusion (CSII) treatment. CSII was initiated in 24 patients aged 55±12 years, without diabetes history, receiving tacrolimus. The mean daily insulin lispro dose was 9.2±5.2 IU. 2.3±1.1% of the total insulin dose were administered between 00:00 and 6:00, 19.5±11.6% between 6:00 and 12:00, 62.3±15.6% between 12:00 and 18:00 and 15.9±9.1% between 18:00 and 24:00. Additional bolus injections were necessary in five patients. Mild hypoglycemia (52-60 mg/dL) occurred in two patients. During the first post-operative week glucose control in CSII patients was overall superior compared to standard-of-care as well as once-daily insulin isophane for fasting and post-supper glucose. We present an algorithm for CSII treatment in kidney transplant recipients, demonstrating similar safety and superior short-term efficacy compared to standard-of-care and once-daily insulin isophane.
Conflict of interest statement
Figures

References
-
- USRDS. US Renal Data System, Annual Report 2011. http://www.usrds.org/2011/view/v2_07.asp 2011.
-
- Chakkera HA, Weil EJ, Castro J, Heilman RL, Reddy KS, Mazur MJ, et al. Hyperglycemia during the immediate period after kidney transplantation. Clin J Am Soc Nephrol. 2009;4(4):853–9. Epub 2009/04/03. doi: CJN.05471008 [pii] doi: 10.2215/CJN.05471008 . - DOI - PMC - PubMed
-
- Hecking M, Werzowa J, Haidinger M, Horl WH, Pascual J, Budde K, et al. Novel views on new-onset diabetes after transplantation: development, prevention and treatment. Nephrol Dial Transplant. 2013;28(3):550–66. Epub 2013/01/19. doi: 10.1093/ndt/gfs583 . - DOI - PMC - PubMed
-
- Chakkera HA, Knowler WC, Devarapalli Y, Weil EJ, Heilman RL, Dueck A, et al. Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin J Am Soc Nephrol. 2010;5(9):1669–75. Epub 2010/06/19. doi: 10.2215/CJN.09481209 ; PubMed Central PMCID: PMC2974410. - DOI - PMC - PubMed
-
- Hecking M, Haidinger M, Doller D, Werzowa J, Tura A, Zhang J, et al. Early Basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol. 2012;23(4):739–49. Epub 2012/02/22. doi: ASN.2011080835 [pii] doi: 10.1681/ASN.2011080835 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

