STAT3 precedes HIF1α transcriptional responses to oxygen and oxygen and glucose deprivation in human brain pericytes
- PMID: 29518129
- PMCID: PMC5843348
- DOI: 10.1371/journal.pone.0194146
STAT3 precedes HIF1α transcriptional responses to oxygen and oxygen and glucose deprivation in human brain pericytes
Abstract
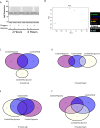
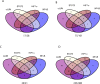
Brain pericytes are important to maintain vascular integrity of the neurovascular unit under both physiological and ischemic conditions. Ischemic stroke is known to induce an inflammatory and hypoxic response due to the lack of oxygen and glucose in the brain tissue. How this early response to ischemia is molecularly regulated in pericytes is largely unknown and may be of importance for future therapeutic targets. Here we evaluate the transcriptional responses in in vitro cultured human brain pericytes after oxygen and/or glucose deprivation. Hypoxia has been widely known to stabilise the transcription factor hypoxia inducible factor 1-alpha (HIF1α) and mediate the induction of hypoxic transcriptional programs after ischemia. However, we find that the transcription factors Jun Proto-Oncogene (c-JUN), Nuclear Factor Of Kappa Light Polypeptide Gene Enhancer In B-Cells (NFκB) and signal transducer and activator of transcription 3 (STAT3) bind genes regulated after 2hours (hs) of omitted glucose and oxygen before HIF1α. Potent HIF1α responses require 6hs of hypoxia to substantiate transcriptional regulation comparable to either c-JUN or STAT3. Phosphorylated STAT3 protein is at its highest after 5 min of oxygen and glucose (OGD) deprivation, whereas maximum HIF1α stabilisation requires 120 min. We show that STAT3 regulates angiogenic and metabolic pathways before HIF1α, suggesting that HIF1α is not the initiating trans-acting factor in the response of pericytes to ischemia.
Conflict of interest statement
Figures




References
-
- Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173–185. doi: 10.1124/pr.57.2.4 - DOI - PubMed
-
- Sweeney MD, Ayyadurai S, Zlokovic BV (2016) Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19: 771–783. doi: 10.1038/nn.4288 - DOI - PMC - PubMed
-
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68: 409–427. doi: 10.1016/j.neuron.2010.09.043 - DOI - PMC - PubMed
-
- Winkler EA, Bell RD, Zlokovic BV (2011) Lack of Smad or Notch leads to a fatal game of brain pericyte hopscotch. Dev Cell 20: 279–280. doi: 10.1016/j.devcel.2011.03.002 - DOI - PubMed
-
- Beckman JD, Grazul-Bilska AT, Johnson ML, Reynolds LP, Redmer DA (2006) Isolation and characterization of ovine luteal pericytes and effects of nitric oxide on pericyte expression of angiogenic factors. Endocrine 29: 467–476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

