Relevance of the protein macrodipole in the membrane-binding process. Interactions of fatty-acid binding proteins with cationic lipid membranes
- PMID: 29518146
- PMCID: PMC5843346
- DOI: 10.1371/journal.pone.0194154
Relevance of the protein macrodipole in the membrane-binding process. Interactions of fatty-acid binding proteins with cationic lipid membranes
Abstract
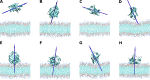
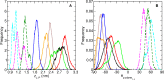
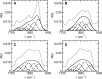
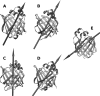
The fatty acid-binding proteins L-BABP and Rep1-NCXSQ bind to anionic lipid membranes by electrostatic interactions. According to Molecular Dynamics (MD) simulations, the interaction of the protein macrodipole with the membrane electric field is a driving force for protein binding and orientation in the interface. To further explore this hypothesis, we studied the interactions of these proteins with cationic lipid membranes. As in the case of anionic lipid membranes, we found that both proteins, carrying a negative as well as a positive net charge, were bound to the positively charged membrane. Their major axis, those connecting the bottom of the β-barrel with the α-helix portal domain, were rotated about 180 degrees as compared with their orientations in the anionic lipid membranes. Fourier transform infrared (FTIR) spectroscopy of the proteins showed that the positively charged membranes were also able to induce conformational changes with a reduction of the β-strand proportion and an increase in α-helix secondary structure. Fatty acid-binding proteins (FABPs) are involved in several cell processes, such as maintaining lipid homeostasis in cells. They transport hydrophobic molecules in aqueous medium and deliver them into lipid membranes. Therefore, the interfacial orientation and conformation, both shown herein to be electrostatically determined, have a strong correlation with the specific mechanism by which each particular FABP exerts its biological function.
Conflict of interest statement
Figures




Similar articles
-
Interactions of the fatty acid-binding protein ReP1-NCXSQ with lipid membranes. Influence of the membrane electric field on binding and orientation.Biochim Biophys Acta. 2014 Mar;1838(3):910-20. doi: 10.1016/j.bbamem.2013.11.008. Epub 2013 Nov 19. Biochim Biophys Acta. 2014. PMID: 24269200
-
Conformational changes, from β-strand to α-helix, of the fatty acid-binding protein ReP1-NCXSQ in anionic lipid membranes: dependence with the vesicle curvature.Eur Biophys J. 2018 Mar;47(2):165-177. doi: 10.1007/s00249-017-1243-5. Epub 2017 Jul 27. Eur Biophys J. 2018. PMID: 28752207
-
Binding and interactions of L-BABP to lipid membranes studied by molecular dynamic simulations.Biochim Biophys Acta. 2008 Jun;1778(6):1390-7. doi: 10.1016/j.bbamem.2008.02.015. Epub 2008 Mar 8. Biochim Biophys Acta. 2008. PMID: 18407826
-
Metabolic regulation of the squid nerve Na(+)/Ca (2+) exchanger: recent developments.Adv Exp Med Biol. 2013;961:149-61. doi: 10.1007/978-1-4614-4756-6_13. Adv Exp Med Biol. 2013. PMID: 23224877 Review.
-
Determinants of specificity at the protein-lipid interface in membranes.FEBS Lett. 2010 May 3;584(9):1713-20. doi: 10.1016/j.febslet.2009.12.060. Epub 2010 Jan 19. FEBS Lett. 2010. PMID: 20085759 Review.
Cited by
-
Effects of Membrane and Biological Target on the Structural and Allosteric Properties of Recoverin: A Computational Approach.Int J Mol Sci. 2019 Oct 10;20(20):5009. doi: 10.3390/ijms20205009. Int J Mol Sci. 2019. PMID: 31658639 Free PMC article.
-
Structure and dynamics of a human myelin protein P2 portal region mutant indicate opening of the β barrel in fatty acid binding proteins.BMC Struct Biol. 2018 Jun 25;18(1):8. doi: 10.1186/s12900-018-0087-2. BMC Struct Biol. 2018. PMID: 29940944 Free PMC article.
-
Specific interactions of peripheral membrane proteins with lipids: what can molecular simulations show us?Biosci Rep. 2022 Apr 29;42(4):BSR20211406. doi: 10.1042/BSR20211406. Biosci Rep. 2022. PMID: 35297484 Free PMC article.
References
-
- Scott AM, Antal CE, Newton AC. Electrostatic and hydrophobic interactions differentially tune membrane binding kinetics of the C2 domain of protein kinase Cα. J Biol Chem. 2013;288: 16905–15. doi: 10.1074/jbc.M113.467456 . - DOI - PMC - PubMed
-
- Heimburg T, Angerstein B, Marsh D. Binding of peripheral proteins to mixed lipid membranes: effect of lipid demixing upon binding. Biophys J. 1999;76: 2575–86. doi: 10.1016/S0006-3495(99)77410-2 . - DOI - PMC - PubMed
-
- Johnson JE, Xie M, Singh LM, Edge R, Cornell RB. Both acidic and basic amino acids in an amphitropic enzyme, CTP:phosphocholine cytidylyltransferase, dictate its selectivity for anionic membranes. J Biol Chem. 2003;278: 514–22. doi: 10.1074/jbc.M206072200 . - DOI - PubMed
-
- Tzlil S, Murray D, Ben-Shaul A. The "electrostatic-switch" mechanism: Monte Carlo study of MARCKS-membrane interaction. Biophys J. 2008;95: 1745–57. doi: 10.1529/biophysj.108.132522 . - DOI - PMC - PubMed
-
- Mulgrew-Nesbitt A, Diraviyam K, Wang J, Singh S, Murray P, Li Z et al. The role of electrostatics in protein-membrane interactions. Biochim Biophys Acta. 2006;1761: 812–26. doi: 10.1016/j.bbalip.2006.07.002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

