The clinical trials landscape for glioblastoma: is it adequate to develop new treatments?
- PMID: 29518210
- PMCID: PMC6280141
- DOI: 10.1093/neuonc/noy027
The clinical trials landscape for glioblastoma: is it adequate to develop new treatments?
Abstract
Background: There have been few treatment advances for patients with glioblastoma (GBM) despite increasing scientific understanding of the disease. While factors such as intrinsic tumor biology and drug delivery are challenges to developing efficacious therapies, it is unclear whether the current clinical trial landscape is optimally evaluating new therapies and biomarkers.
Methods: We queried ClinicalTrials.gov for interventional clinical trials for patients with GBM initiated between January 2005 and December 2016 and abstracted data regarding phase, status, start and end dates, testing locations, endpoints, experimental interventions, sample size, clinical presentation/indication, and design to better understand the clinical trials landscape.
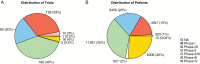
Results: Only approximately 8%-11% of patients with newly diagnosed GBM enroll on clinical trials with a similar estimate for all patients with GBM. Trial duration was similar across phases with median time to completion between 3 and 4 years. While 93% of clinical trials were in phases I-II, 26% of the overall clinical trial patient population was enrolled on phase III studies. Of the 8 completed phase III trials, only 1 reported positive results. Although 58% of the phase III trials were supported by phase II data with a similar endpoint, only 25% of these phase II trials were randomized.
Conclusions: The clinical trials landscape for GBM is characterized by long development times, inadequate dissemination of information, suboptimal go/no-go decision making, and low patient participation.
Figures
Comment in
-
The trials of neuro-oncology clinical research.Neuro Oncol. 2018 Jul 5;20(8):1007-1008. doi: 10.1093/neuonc/noy076. Neuro Oncol. 2018. PMID: 29878174 Free PMC article. No abstract available.
-
Disparities along the glioblastoma clinical trials landscape.Neuro Oncol. 2019 Feb 14;21(2):285-286. doi: 10.1093/neuonc/noy176. Neuro Oncol. 2019. PMID: 30476295 Free PMC article. No abstract available.
References
-
- Alexander B, Cloughesy T. Adult glioblastoma. J Clin Oncol. 2017;35(21):2402–2409. - PubMed
-
- Sachs M. An Interface to the Clinicaltrials.Gov API. 2017. https://github.com/sachsmc/rclinicaltrials.
-
- Cihoric N, Tsikkinis A, Minniti G. Current status and perspectives of interventional clinical trials for glioblastoma—analysis of ClinicalTrials.gov. Radiat Oncol. 2017;12(1). doi:10.1186/s13014-016-0740-5. - PMC - PubMed
-
- 110th Congress. Food and Drug Administration Amendments Act of 2007 2007. https://www.gpo.gov/fdsys/pkg/PLAW-110publ85/html/PLAW-110publ85.htm.
-
- Home—ClinicalTrials.gov https://www.clinicaltrials.gov/. Accessed October 31, 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical