A Systems Neuroscience Approach to Migraine
- PMID: 29518355
- PMCID: PMC6402597
- DOI: 10.1016/j.neuron.2018.01.029
A Systems Neuroscience Approach to Migraine
Abstract
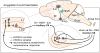
Migraine is an extremely common but poorly understood nervous system disorder. We conceptualize migraine as a disorder of sensory network gain and plasticity, and we propose that this framing makes it amenable to the tools of current systems neuroscience.
Keywords: allodynia; cortical spreading depression; gain; migraine; pain; photophobia; plasticity; systems neuroscience.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures

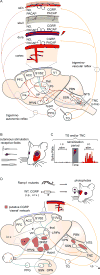

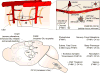
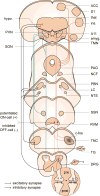
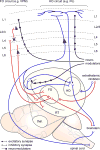

References
-
- Alstadhaug KB. Migraine and the hypothalamus. Cephalalgia. 2009;29:809–17. - PubMed
-
- Ambrosini A, D’Alessio C, Magis D, Schoenen J. Targeting pericranial nerve branches to treat migraine: Current approaches and perspectives. Cephalalgia. 2015;35:1308–1322. - PubMed
-
- Ashina M, Hansen JM, À Dunga BO, Olesen J. Human models of migraine - short-term pain for long-term gain. Nat. Rev. Neurol. 2017;13:713–724. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

