Mast cell activation test in the diagnosis of allergic disease and anaphylaxis
- PMID: 29518421
- PMCID: PMC6075471
- DOI: 10.1016/j.jaci.2018.01.043
Mast cell activation test in the diagnosis of allergic disease and anaphylaxis
Abstract
Background: Food allergy is an increasing public health issue and the most common cause of life-threatening anaphylactic reactions. Conventional allergy tests assess for the presence of allergen-specific IgE, significantly overestimating the rate of true clinical allergy and resulting in overdiagnosis and adverse effect on health-related quality of life.
Objective: To undertake initial validation and assessment of a novel diagnostic tool, we used the mast cell activation test (MAT).
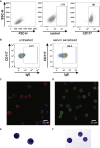
Methods: Primary human blood-derived mast cells (MCs) were generated from peripheral blood precursors, sensitized with patients' sera, and then incubated with allergen. MC degranulation was assessed by means of flow cytometry and mediator release. We compared the diagnostic performance of MATs with that of existing diagnostic tools to assess in a cohort of peanut-sensitized subjects undergoing double-blind, placebo-controlled challenge.
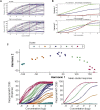
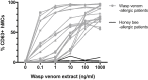
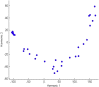
Results: Human blood-derived MCs sensitized with sera from patients with peanut, grass pollen, and Hymenoptera (wasp venom) allergy demonstrated allergen-specific and dose-dependent degranulation, as determined based on both expression of surface activation markers (CD63 and CD107a) and functional assays (prostaglandin D2 and β-hexosaminidase release). In this cohort of peanut-sensitized subjects, the MAT was found to have superior discrimination performance compared with other testing modalities, including component-resolved diagnostics and basophil activation tests. Using functional principle component analysis, we identified 5 clusters or patterns of reactivity in the resulting dose-response curves, which at preliminary analysis corresponded to the reaction phenotypes seen at challenge.
Conclusion: The MAT is a robust tool that can confer superior diagnostic performance compared with existing allergy diagnostics and might be useful to explore differences in effector cell function between basophils and MCs during allergic reactions.
Keywords: Anaphylaxis; basophil activation test; diagnosis; food allergy; mast cell activation test; mast cells; peanut allergy.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures














Comment in
-
Reply.J Allergy Clin Immunol. 2018 Sep;142(3):1019. doi: 10.1016/j.jaci.2018.06.021. Epub 2018 Jul 25. J Allergy Clin Immunol. 2018. PMID: 30055830 No abstract available.
-
Mast cell activation test versus basophil activation test and related competing issues.J Allergy Clin Immunol. 2018 Sep;142(3):1018-1019. doi: 10.1016/j.jaci.2018.06.020. Epub 2018 Jul 25. J Allergy Clin Immunol. 2018. PMID: 30055831 No abstract available.
References
-
- Nwaru B.I., Hickstein L., Panesar S.S., Roberts G., Muraro A., Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992–1007. - PubMed
-
- Turner P.J., Boyle R.J. Food allergy in children: what is new? Curr Opin Clin Nutr Metab Care. 2014;17:285–293. - PubMed
-
- Sampson H.A., Gerth van Wijk R., Bindslev-Jensen C., Sicherer S., Teuber S.S., Burks A.W. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130:1260–1274. - PubMed
-
- Statement by the American Academy of Allergy, Asthma & Immunology, American College of Allergy, Asthma & Immunology and the Canadian Society of Allergy and Clinical Immunology relating to fatality during food challenge test. http://csaci.ca/wp-content/uploads/2017/08/Statement-to-public-regarding... Available at:
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

