Characterization and validation of Entamoeba histolytica pantothenate kinase as a novel anti-amebic drug target
- PMID: 29518650
- PMCID: PMC6114107
- DOI: 10.1016/j.ijpddr.2018.02.004
Characterization and validation of Entamoeba histolytica pantothenate kinase as a novel anti-amebic drug target
Abstract
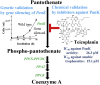
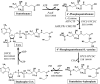
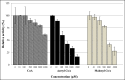
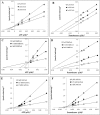
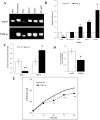
The Coenzyme A (CoA), as a cofactor involved in >100 metabolic reactions, is essential to the basic biochemistry of life. Here, we investigated the CoA biosynthetic pathway of Entamoeba histolytica (E. histolytica), an enteric protozoan parasite responsible for human amebiasis. We identified four key enzymes involved in the CoA pathway: pantothenate kinase (PanK, EC 2.7.1.33), bifunctional phosphopantothenate-cysteine ligase/decarboxylase (PPCS-PPCDC), phosphopantetheine adenylyltransferase (PPAT) and dephospho-CoA kinase (DPCK). Cytosolic enzyme PanK, was selected for further biochemical, genetic, and phylogenetic characterization. Since E. histolytica PanK (EhPanK) is physiologically important and sufficiently divergent from its human orthologs, this enzyme represents an attractive target for the development of novel anti-amebic chemotherapies. Epigenetic gene silencing of PanK resulted in a significant reduction of PanK activity, intracellular CoA concentrations, and growth retardation in vitro, reinforcing the importance of this gene in E. histolytica. Furthermore, we screened the Kitasato Natural Products Library for inhibitors of recombinant EhPanK, and identified 14 such compounds. One compound demonstrated moderate inhibition of PanK activity and cell growth at a low concentration, as well as differential toxicity towards E. histolytica and human cells.
Keywords: Amebiasis; Coenzyme A; Drug development; Entamoeba histolytica; Gene silencing; Pantothenate kinase.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Biochemical, Metabolomic, and Genetic Analyses of Dephospho Coenzyme A Kinase Involved in Coenzyme A Biosynthesis in the Human Enteric Parasite Entamoeba histolytica.Front Microbiol. 2018 Nov 30;9:2902. doi: 10.3389/fmicb.2018.02902. eCollection 2018. Front Microbiol. 2018. PMID: 30555442 Free PMC article.
-
Discovery of Antiamebic Compounds That Inhibit Cysteine Synthase From the Enteric Parasitic Protist Entamoeba histolytica by Screening of Microbial Secondary Metabolites.Front Cell Infect Microbiol. 2018 Dec 5;8:409. doi: 10.3389/fcimb.2018.00409. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30568921 Free PMC article.
-
Identification and characterization of archaeal-type FAD synthase as a novel tractable drug target from the parasitic protozoa Entamoeba histolytica.mSphere. 2024 Sep 25;9(9):e0034724. doi: 10.1128/msphere.00347-24. Epub 2024 Aug 27. mSphere. 2024. PMID: 39189775 Free PMC article.
-
Entamoeba thiol-based redox metabolism: A potential target for drug development.Mol Biochem Parasitol. 2016 Mar-Apr;206(1-2):39-45. doi: 10.1016/j.molbiopara.2016.01.004. Epub 2016 Jan 13. Mol Biochem Parasitol. 2016. PMID: 26775086 Review.
-
Flavonoids as a Natural Treatment Against Entamoeba histolytica.Front Cell Infect Microbiol. 2018 Jun 22;8:209. doi: 10.3389/fcimb.2018.00209. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29988403 Free PMC article. Review.
Cited by
-
Entamoeba histolytica "mutator" strain with a high rate of genetic mutations assists the elucidation of drug resistance mechanisms.Microbiol Spectr. 2025 Aug 5;13(8):e0121025. doi: 10.1128/spectrum.01210-25. Epub 2025 Jun 12. Microbiol Spectr. 2025. PMID: 40503832 Free PMC article.
-
Revisiting Drug Development Against the Neglected Tropical Disease, Amebiasis.Front Cell Infect Microbiol. 2021 Feb 24;10:628257. doi: 10.3389/fcimb.2020.628257. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33718258 Free PMC article. Review.
-
Biochemical, Metabolomic, and Genetic Analyses of Dephospho Coenzyme A Kinase Involved in Coenzyme A Biosynthesis in the Human Enteric Parasite Entamoeba histolytica.Front Microbiol. 2018 Nov 30;9:2902. doi: 10.3389/fmicb.2018.02902. eCollection 2018. Front Microbiol. 2018. PMID: 30555442 Free PMC article.
-
Glycerol biosynthetic pathway plays an essential role in proliferation and antioxidative defense in the human enteric protozoan parasite Entamoeba histolytica.Sci Rep. 2023 Sep 5;13(1):14596. doi: 10.1038/s41598-023-40670-z. Sci Rep. 2023. PMID: 37669981 Free PMC article.
-
Antineoplastic kinase inhibitors: A new class of potent anti-amoebic compounds.PLoS Negl Trop Dis. 2021 Feb 8;15(2):e0008425. doi: 10.1371/journal.pntd.0008425. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33556060 Free PMC article.
References
-
- Abiko Y. Metabolic Pathways. Academic Press; New York: 1975. Metabolism of coenzyme a; pp. 1–25.
-
- Awasthy D., Ambady A., Bhat J., Sheikh G., Ravishankar S., Subbulakshmi V., Mukherjee K., Sambandamurthy V., Sharma U. Essentiality and functional analysis of type I and type III pantothenate kinases of Mycobacterium tuberculosis. Microbiology. 2010;156:2691–2701. - PubMed
-
- Begley T.P., Kinsland C., Strauss E. The biosynthesis of coenzyme A in bacteria. Vitam. Horm. 2001;61:157–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

