Fatigue loading of tendon results in collagen kinking and denaturation but does not change local tissue mechanics
- PMID: 29519673
- PMCID: PMC6590695
- DOI: 10.1016/j.jbiomech.2018.02.014
Fatigue loading of tendon results in collagen kinking and denaturation but does not change local tissue mechanics
Abstract
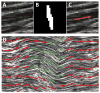
Fatigue loading is a primary cause of tendon degeneration, which is characterized by the disruption of collagen fibers and the appearance of abnormal (e.g., cartilaginous, fatty, calcified) tissue deposits. The formation of such abnormal deposits, which further weakens the tissue, suggests that resident tendon cells acquire an aberrant phenotype in response to fatigue damage and the resulting altered mechanical microenvironment. While fatigue loading produces clear changes in collagen organization and molecular denaturation, no data exist regarding the effect of fatigue on the local tissue mechanical properties. Therefore, the objective of this study was to identify changes in the local tissue stiffness of tendons after fatigue loading. We hypothesized that fatigue damage would reduce local tissue stiffness, particularly in areas with significant structural damage (e.g., collagen denaturation). We tested this hypothesis by identifying regions of local fatigue damage (i.e., collagen fiber kinking and molecular denaturation) via histologic imaging and by measuring the local tissue modulus within these regions via atomic force microscopy (AFM). Counter to our initial hypothesis, we found no change in the local tissue modulus as a consequence of fatigue loading, despite widespread fiber kinking and collagen denaturation. These data suggest that immediate changes in topography and tissue structure - but not local tissue mechanics - initiate the early changes in tendon cell phenotype as a consequence of fatigue loading that ultimately culminate in tendon degeneration.
Keywords: Atomic force microscopy; Fatigue; Microscale mechanics; Second harmonic generation imaging; Tendon.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the authors have any conflicts of interest to disclose.
Figures





Similar articles
-
Mouse Achilles tendons exhibit collagen disorganization but minimal collagen denaturation during cyclic loading to failure.J Biomech. 2023 Apr;151:111545. doi: 10.1016/j.jbiomech.2023.111545. Epub 2023 Mar 12. J Biomech. 2023. PMID: 36944295 Free PMC article.
-
The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon.J Biomech. 2012 Jan 3;45(1):59-65. doi: 10.1016/j.jbiomech.2011.10.008. Epub 2011 Nov 4. J Biomech. 2012. PMID: 22055428 Free PMC article.
-
Tendons exhibit greater resistance to tissue and molecular-level damage with increasing strain rate during cyclic fatigue.Acta Biomater. 2021 Oct 15;134:435-442. doi: 10.1016/j.actbio.2021.07.045. Epub 2021 Jul 24. Acta Biomater. 2021. PMID: 34314889 Free PMC article.
-
In vivo investigation of tendon responses to mechanical loading.J Musculoskelet Neuronal Interact. 2011 Jun;11(2):115-23. J Musculoskelet Neuronal Interact. 2011. PMID: 21625048 Review.
-
The interface of mechanical loading and biological variables as they pertain to the development of tendinosis.J Musculoskelet Neuronal Interact. 2011 Jun;11(2):94-105. J Musculoskelet Neuronal Interact. 2011. PMID: 21625046 Review.
Cited by
-
The LINC Complex Regulates Tendon Elastic Modulus, Collagen Crimp, and Lateral Expansion During Early Postnatal Development.J Orthop Res. 2025 Jun;43(6):1090-1100. doi: 10.1002/jor.26069. Epub 2025 Mar 16. J Orthop Res. 2025. PMID: 40089904 Free PMC article.
-
Optogenetic-Induced Muscle Loading Leads to Mechanical Adaptation of the Achilles Tendon Enthesis in Mice.bioRxiv [Preprint]. 2023 May 2:2023.04.11.536376. doi: 10.1101/2023.04.11.536376. bioRxiv. 2023. Update in: Sci Adv. 2023 Jun 23;9(25):eadf4683. doi: 10.1126/sciadv.adf4683. PMID: 37090593 Free PMC article. Updated. Preprint.
-
Optogenetic-induced muscle loading leads to mechanical adaptation of the Achilles tendon enthesis in mice.Sci Adv. 2023 Jun 23;9(25):eadf4683. doi: 10.1126/sciadv.adf4683. Epub 2023 Jun 23. Sci Adv. 2023. PMID: 37352350 Free PMC article.
-
Aged Tendons Exhibit Altered Mechanisms of Strain-Dependent Extracellular Matrix Remodeling.J Biomech Eng. 2024 Jul 1;146(7):071009. doi: 10.1115/1.4065270. J Biomech Eng. 2024. PMID: 38584416 Free PMC article.
-
Three-dimensional computation of fibre orientation, diameter and branching in segmented image stacks of fibrous networks.J R Soc Interface. 2020 Aug;17(169):20200371. doi: 10.1098/rsif.2020.0371. Epub 2020 Aug 5. J R Soc Interface. 2020. PMID: 32752994 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

