Autophagy balances mtDNA synthesis and degradation by DNA polymerase POLG during starvation
- PMID: 29519802
- PMCID: PMC5940314
- DOI: 10.1083/jcb.201801168
Autophagy balances mtDNA synthesis and degradation by DNA polymerase POLG during starvation
Abstract
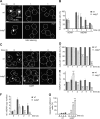
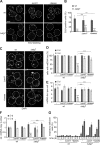
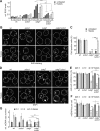
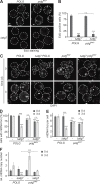
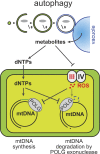
Mitochondria contain tens to thousands of copies of their own genome (mitochondrial DNA [mtDNA]), creating genetic redundancy capable of buffering mutations in mitochondrial genes essential for cellular function. However, the mechanisms regulating mtDNA copy number have been elusive. Here we found that DNA synthesis and degradation by mtDNA polymerase γ (POLG) dynamically controlled mtDNA copy number in starving yeast cells dependent on metabolic homeostasis provided by autophagy. Specifically, the continuous mtDNA synthesis by POLG in starving wild-type cells was inhibited by nucleotide insufficiency and elevated mitochondria-derived reactive oxygen species in the presence of autophagy dysfunction. Moreover, after prolonged starvation, 3'-5' exonuclease-dependent mtDNA degradation by POLG adjusted the initially increasing mtDNA copy number in wild-type cells, but caused quantitative mtDNA instability and irreversible respiratory dysfunction in autophagy-deficient cells as a result of nucleotide limitations. In summary, our study reveals that mitochondria rely on the homeostatic functions of autophagy to balance synthetic and degradative modes of POLG, which control copy number dynamics and stability of the mitochondrial genome.
© 2018 Medeiros et al.
Figures
Comment in
-
Autophagy determines mtDNA copy number dynamics during starvation.Autophagy. 2019 Jan;15(1):178-179. doi: 10.1080/15548627.2018.1532263. Epub 2018 Oct 13. Autophagy. 2019. PMID: 30301401 Free PMC article.
References
-
- Bratic A., Kauppila T.E., Macao B., Grönke S., Siibak T., Stewart J.B., Baggio F., Dols J., Partridge L., Falkenberg M., et al. 2015. Complementation between polymerase- and exonuclease-deficient mitochondrial DNA polymerase mutants in genomically engineered flies. Nat. Commun. 6:8808 10.1038/ncomms9808 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

