Nonadherence in the era of severe asthma biologics and thermoplasty
- PMID: 29519922
- PMCID: PMC5884695
- DOI: 10.1183/13993003.01836-2017
Nonadherence in the era of severe asthma biologics and thermoplasty
Abstract
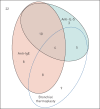
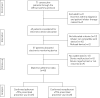
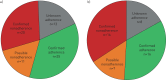
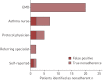
Nonadherence to inhaled preventers impairs asthma control. Electronic monitoring devices (EMDs) can objectively measure adherence. Their use has not been reported in difficult asthma patients potentially suitable for novel therapies, i.e. biologics and bronchial thermoplasty.Consecutive patients with difficult asthma were assessed for eligibility for novel therapies. Medication adherence, defined as taking >75% of prescribed doses, was assessed by EMD and compared with standardised clinician assessment over an 8-week period.Among 69 difficult asthma patients, adherence could not be analysed in 13, due to device incompatibility or malfunction. Nonadherence was confirmed in 20 out of 45 (44.4%) patients. Clinical assessment of nonadherence was insensitive (physician 15%, nurse 28%). Serum eosinophils were higher in nonadherent patients. Including 11 patients with possible nonadherence (device refused or not returned) increased the nonadherence rate to 31 out of 56 (55%) patients. Severe asthma criteria were fulfilled by 59 out of 69 patients. 47 were eligible for novel therapies, with confirmed nonadherence in 16 out of 32 (50%) patients with EMD data; including seven patients with possible nonadherence increased the nonadherence rate to 23 out of 39 (59%).At least half the patients eligible for novel therapies were nonadherent to preventers. Nonadherence was often undetectable by clinical assessments. Preventer adherence must be confirmed objectively before employing novel severe asthma therapies.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: M. Hew received an unrestricted grant from GlaxoSmithKline (GSK) during the conduct of the study to purchase adherence monitoring devices (GSK had no role or input into the design, conduct, analysis or reporting of the study); and has received grants from AstraZeneca (unrestricted grant to develop an electronic clinic template) and Novartis (unrestricted grant to host a severe asthma preceptorship), personal fees for advisory board participation from GSK, Seqirus and AstraZeneca; and has undertaken contracted research on behalf of AstraZeneca, Novartis, GSK and Sanofi, outside the submitted work; all for which his employer Alfred Health has been reimbursed.
Figures
Comment in
-
Nonadherence with inhaled preventer therapy in severe asthmatic patients on long-term omalizumab.Eur Respir J. 2018 Aug 16;52(2):1801025. doi: 10.1183/13993003.01025-2018. Print 2018 Aug. Eur Respir J. 2018. PMID: 29976649 No abstract available.
References
-
- Hekking P-PW, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. - PubMed
-
- Hew M, Chung KF. Corticosteroid insensitivity in severe asthma: significance, mechanisms and aetiology. Intern Med J 2010; 40: 323–334. - PubMed
-
- Bousquet J, Siergiejko Z, Świebocka E, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy 2011; 66: 671–678. - PubMed
-
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
