Key design considerations for adaptive clinical trials: a primer for clinicians
- PMID: 29519932
- PMCID: PMC5842365
- DOI: 10.1136/bmj.k698
Key design considerations for adaptive clinical trials: a primer for clinicians
Abstract
This article reviews important considerations for researchers who are designing adaptive clinical trials. These differ from conventional clinical trials because they allow and even enforce continual modifications to key components of trial design while data are being collected. This innovative approach has the potential to reduce resource use, decrease time to trial completion, limit allocation of participants to inferior interventions, and improve the likelihood that trial results will be scientifically or clinically relevant. Adaptive designs have mostly been used in trials evaluating drugs, but their use is spreading. The US Food and Drug Administration recently issued guidance on adaptive trial designs, which highlighted general principles and different types of adaptive clinical trials but did not provide concrete guidance about important considerations in designing such trials. Decisions to adapt a trial are not arbitrary; they are based on decision rules that have been rigorously examined via statistical simulations before the first trial participant is enrolled. The authors review important characteristics of adaptive trials and common types of study modifications and provide a practical guide, illustrated with a case study, to aid investigators who are planning an adaptive clinical trial
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Figures
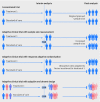


References
-
- European Medicines Agency. Adaptive pathways 2017 http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/gen....
-
- United States Food and Drug Administration. Adaptive designs for medical device clinical studies., 2016. https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
