Disease-induced assemblage of a plant-beneficial bacterial consortium
- PMID: 29520025
- PMCID: PMC5956071
- DOI: 10.1038/s41396-018-0093-1
Disease-induced assemblage of a plant-beneficial bacterial consortium
Abstract
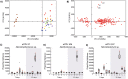
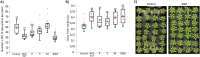
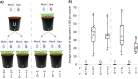
Disease suppressive soils typically develop after a disease outbreak due to the subsequent assembly of protective microbiota in the rhizosphere. The role of the plant immune system in the assemblage of a protective rhizosphere microbiome is largely unknown. In this study, we demonstrate that Arabidopsis thaliana specifically promotes three bacterial species in the rhizosphere upon foliar defense activation by the downy mildew pathogen Hyaloperonospora arabidopsidis. The promoted bacteria were isolated and found to interact synergistically in biofilm formation in vitro. Although separately these bacteria did not affect the plant significantly, together they induced systemic resistance against downy mildew and promoted growth of the plant. Moreover, we show that the soil-mediated legacy of a primary population of downy mildew infected plants confers enhanced protection against this pathogen in a second population of plants growing in the same soil. Together our results indicate that plants can adjust their root microbiome upon pathogen infection and specifically recruit a group of disease resistance-inducing and growth-promoting beneficial microbes, therewith potentially maximizing the chance of survival of their offspring that will grow in the same soil.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Van der Putten WH, Van Dijk C, Peters BAM. Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature. 1993;362:53–56. doi: 10.1038/362053a0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

