Histone deacetylases mediate GABAA receptor expression, physiology, and behavioral maladaptations in rat models of alcohol dependence
- PMID: 29520058
- PMCID: PMC5983537
- DOI: 10.1038/s41386-018-0034-8
Histone deacetylases mediate GABAA receptor expression, physiology, and behavioral maladaptations in rat models of alcohol dependence
Abstract
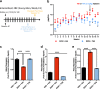
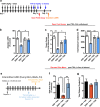
Alcohol use disorders are chronic debilitating diseases characterized by severe withdrawal symptoms that contribute to morbidity and relapse. GABAA receptor (GABAAR) adaptations have long been implicated in the chronic effects of alcohol and contribute to many withdrawal symptoms associated with alcohol dependence. In rodents, GABAAR hypofunction results from decreases in Gabra1 expression, although the underlying mechanism controlling Gabra1 expression after chronic ethanol exposure is still unknown. We found that chronic ethanol exposure using either ethanol gavage or two-bottle choice voluntary access paradigms decreased Gabra1 expression and increased Hdac2 and Hdac3 expression. Administration of the HDAC inhibitor trichostatin A (TSA) after chronic ethanol exposure prevents the decrease in Gabra1 expression and function as well as the increase in Hdac2 and Hdac3 expression in both the cortex and the medial prefrontal cortex (mPFC). Chronic ethanol exposure and withdrawal, but not acute ethanol exposure or acute withdrawal, cause a selective upregulation of HDAC2 and HDAC3 associated with the Gabra1 promoter that accompanies a decrease in H3 acetylation of the Gabra1 promoter and the reduction in GABAAR α1 subunit expression. TSA administration prevented each of these molecular events as well as behavioral manifestations of ethanol dependence, including tolerance to zolpidem-induced loss of righting reflex, reduced open-arm time in the elevated plus maze, reduced center-time and locomotor activity in the open-field assay, and TSA reduced voluntary ethanol consumption. The results show how chronic ethanol exposure regulates the highly prominent GABAAR α1 subunit by an epigenetic mechanism that represents a potential treatment modality for alcohol dependence.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Miller NS, Gold MS. Management of withdrawal syndromes and relapse prevention in drug and alcohol dependence. Am Fam Physician. 1998;58:139–46. - PubMed
-
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

