Mice with reduced DAT levels recreate seasonal-induced switching between states in bipolar disorder
- PMID: 29520059
- PMCID: PMC6006292
- DOI: 10.1038/s41386-018-0031-y
Mice with reduced DAT levels recreate seasonal-induced switching between states in bipolar disorder
Abstract
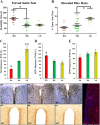
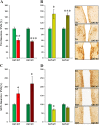
Developing novel therapeutics for bipolar disorder (BD) has been hampered by limited mechanistic knowledge how sufferers switch between mania and depression-how the same brain can switch between extreme states-described as the "holy grail" of BD research. Strong evidence implicates seasonally-induced switching between states, with mania associated with summer-onset, depression with winter-onset. Determining mechanisms of and sensitivity to such switching is required. C57BL/6J and dopamine transporter hypomorphic (DAT-HY 50% expression) mice performed a battery of psychiatry-relevant behavioral tasks following 2-week housing in chambers under seasonally relevant photoperiod extremes. Summer-like and winter-like photoperiod exposure induced mania-relevant and depression-relevant behaviors respectively in mice. This behavioral switch paralleled neurotransmitter switching from dopamine to somatostatin in hypothalamic neurons (receiving direct input from the photoperiod-processing center, the suprachiasmatic nucleus). Mice with reduced DAT expression exhibited hypersensitivity to these summer-like and winter-like photoperiods, including more extreme mania-relevant (including reward sensitivity during reinforcement learning), and depression-relevant (including punishment-sensitivity and loss-sensitivity during reinforcement learning) behaviors. DAT mRNA levels switched in wildtype littermate mice across photoperiods, an effect not replicated in DAT hypomorphic mice. This inability to adjust DAT levels to match photoperiod-induced neurotransmitter switching as a homeostatic control likely contributes to the susceptibility of DAT hypormophic mice to these switching photoperiods. These data reveal the potential contribution of photoperiod-induced neuroplasticity within an identified circuit of the hypothalamus, linked with reduced DAT function, underlying switching between states in BD. Further investigations of the circuit will likely identify novel therapeutic targets to block switching between states.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Comment in
-
Evidence for light-entrainment-induced switching between depression- & mania-relevant behaviors in mice.Neuropsychopharmacology. 2019 Sep;44(10):1679-1680. doi: 10.1038/s41386-019-0338-3. Epub 2019 Feb 20. Neuropsychopharmacology. 2019. PMID: 30787425 Free PMC article. No abstract available.
-
Switching winter and summer photoperiods in an animal model of bipolar disorder.Neuropsychopharmacology. 2019 Sep;44(10):1677-1678. doi: 10.1038/s41386-019-0337-4. Epub 2019 Feb 20. Neuropsychopharmacology. 2019. PMID: 30787426 Free PMC article. No abstract available.
Similar articles
-
The effects of reduced dopamine transporter function and chronic lithium on motivation, probabilistic learning, and neurochemistry in mice: Modeling bipolar mania.Neuropharmacology. 2017 Feb;113(Pt A):260-270. doi: 10.1016/j.neuropharm.2016.07.030. Epub 2016 Oct 11. Neuropharmacology. 2017. PMID: 27732870 Free PMC article.
-
Brexpiprazole reduces hyperactivity, impulsivity, and risk-preference behavior in mice with dopamine transporter knockdown-a model of mania.Psychopharmacology (Berl). 2017 Mar;234(6):1017-1028. doi: 10.1007/s00213-017-4543-7. Epub 2017 Feb 3. Psychopharmacology (Berl). 2017. PMID: 28160035 Free PMC article.
-
Reduced dopamine transporter functioning induces high-reward risk-preference consistent with bipolar disorder.Neuropsychopharmacology. 2014 Dec;39(13):3112-22. doi: 10.1038/npp.2014.170. Epub 2014 Jul 9. Neuropsychopharmacology. 2014. PMID: 25005251 Free PMC article.
-
Investigating the mechanism(s) underlying switching between states in bipolar disorder.Eur J Pharmacol. 2015 Jul 15;759:151-62. doi: 10.1016/j.ejphar.2015.03.019. Epub 2015 Mar 23. Eur J Pharmacol. 2015. PMID: 25814263 Free PMC article. Review.
-
Enlightened: addressing circadian and seasonal changes in photoperiod in animal models of bipolar disorder.Transl Psychiatry. 2021 Jul 5;11(1):373. doi: 10.1038/s41398-021-01494-5. Transl Psychiatry. 2021. PMID: 34226504 Free PMC article. Review.
Cited by
-
Short-active gestational photoperiod reduces effortful choice behavior in mice, partial normalization by d-amphetamine.Psychopharmacology (Berl). 2023 Nov;240(11):2303-2315. doi: 10.1007/s00213-023-06337-3. Epub 2023 Feb 18. Psychopharmacology (Berl). 2023. PMID: 36806900
-
Sleep and circadian disruption in bipolar disorders: From psychopathology to digital phenotyping in clinical practice.Psychiatry Clin Neurosci. 2024 Nov;78(11):654-666. doi: 10.1111/pcn.13729. Epub 2024 Aug 30. Psychiatry Clin Neurosci. 2024. PMID: 39210713 Free PMC article. Review.
-
Seasonal changes in day length induce multisynaptic neurotransmitter switching to regulate hypothalamic network activity and behavior.Sci Adv. 2022 Sep 2;8(35):eabn9867. doi: 10.1126/sciadv.abn9867. Epub 2022 Sep 2. Sci Adv. 2022. PMID: 36054362 Free PMC article.
-
Hypothalamic Oxytocin Neuronal Activation Induces Bipolar-Like Mood Changes in Mice in a Sex- and Dosage-Dependent Manner.Neurosci Bull. 2025 Aug 22. doi: 10.1007/s12264-025-01475-4. Online ahead of print. Neurosci Bull. 2025. PMID: 40847250
-
Converging evidence that short-active photoperiod increases acetylcholine signaling in the hippocampus.Cogn Affect Behav Neurosci. 2020 Dec;20(6):1173-1183. doi: 10.3758/s13415-020-00824-2. Cogn Affect Behav Neurosci. 2020. PMID: 32794101 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

