Factors associated with appropriate inhaler use in patients with COPD - lessons from the REAL survey
- PMID: 29520137
- PMCID: PMC5834182
- DOI: 10.2147/COPD.S149404
Factors associated with appropriate inhaler use in patients with COPD - lessons from the REAL survey
Erratum in
-
Erratum: Factors associated with appropriate inhaler use in patients with COPD - lessons from the REAL survey [Erratum].Int J Chron Obstruct Pulmon Dis. 2018 Jul 25;13:2253-2254. doi: 10.2147/COPD.S178410. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30100714 Free PMC article.
Abstract
Background: Nonadherence to medication and incorrect use of inhalers represent significant barriers to optimal disease management of patients with chronic obstructive pulmonary disease (COPD). Thus, health care professionals (HCPs) play a critical role in educating their patients on appropriate inhaler use and in ensuring medication adherence. However, many patients do not receive appropriate inhaler training or have not had their inhaler technique checked.
Methods: The Real-life Experience and Accuracy of inhaLer use (REAL) survey was a computer-assisted, telephonic survey consisting of 23 questions gathering real-world information on correct inhaler use, inhalation technique, device attributes, adherence, dosing accuracy, training, correct device use, ease of use, and factors that influence patient adherence in commercially available inhalers delivering COPD maintenance therapy. All results are based on patient-reported data.
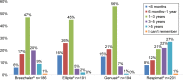
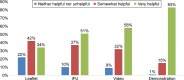
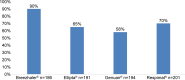
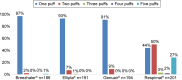
Results: The survey was conducted between January 4, 2016 and February 2, 2016. A total of 764 patients using various inhalers (Breezhaler® =186; Ellipta® =191; Genuair® =194; Respimat® =201) with mild to very severe COPD, with a mean ± SD age 56±9.8 years, completed the survey. Patient self-reported adherence was significantly lower in younger patients compared to older patients (p=0.020). Eighty-three percent of patients indicated that a demonstration (in-person) was "very helpful" versus 58% for video. Patient preferences for training methods were as follows: demonstration of inhaler use (83%), video (58%), instructions for use (51%), and leaflet (34%). Twenty-nine percent of patients had not been checked to see if they were using their device correctly by a HCP within the last two years. Patients who were checked were significantly more adherent than unchecked patients (p=0.020). The majority of the patients using Breezhaler reported either being very confident or confident of having taken a full dose, which was higher than those using Genuair, Ellipta (α=0.05), and Respimat (α=0.05). Treatment adherence in the last 30 days was highest with Breezhaler followed by Respimat, Ellipta, and Genuair.
Conclusion: The REAL survey identified attributes that influenced patient adherence and optimal inhaler use. Predictive attributes that influence patient adherence which HCPs should be aware of include age and disease severity. Modifiable attributes which the HCP can influence include correct inhaler use training, choice of training methods, checking patient inhaler technique at subsequent visits, and device selection. Inhalers are integral in the effective management of patients with COPD; it is therefore important that patients use the inhaler correctly and have full confidence in the dosage.
Keywords: adherence; chronic obstructive pulmonary disease; dose confidence; double dosing; inhaler use; survey.
Conflict of interest statement
Disclosure DLK, VB, and FSG are all employees of Novartis and have received no other funding. MG and SW are employees of GfK Switzerland AG and have received no other funding. Professor David Price has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Mylan, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Teva Pharmaceuticals, Theravance, UK National Health Service, Zentiva; payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma and Novartis; payment for travel/accommodation/meeting expenses from Aerocrine, AstraZeneca, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; funding for patient enrolment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals, and Zentiva; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia, Singapore, and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation Programme, and Health Technology Assessment. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma-COPD overlap syndrome consulting for routine care.Int J Chron Obstruct Pulmon Dis. 2018 Mar 16;13:927-936. doi: 10.2147/COPD.S154525. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29588581 Free PMC article.
-
Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners.J Aerosol Med Pulm Drug Deliv. 2015 Jun;28(3):219-28. doi: 10.1089/jamp.2014.1142. Epub 2014 Sep 29. J Aerosol Med Pulm Drug Deliv. 2015. PMID: 25265316 Free PMC article.
-
Satisfaction, preference and error occurrence of three dry powder inhalers as assessed by a cohort naïve to inhaler operation.Int J Chron Obstruct Pulmon Dis. 2018 Jun 15;13:1949-1963. doi: 10.2147/COPD.S152285. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29942127 Free PMC article. Clinical Trial.
-
Inhaled drug delivery in the hands of the patient.J Aerosol Med Pulm Drug Deliv. 2014 Dec;27(6):414-8. doi: 10.1089/jamp.2014.1132. J Aerosol Med Pulm Drug Deliv. 2014. PMID: 25238005 Review.
-
Requirements, Strengths and Weaknesses of Inhaler Devices for COPD Patients from the Expert Prescribers' Point of View: Results of the EPOCA Delphi Consensus.COPD. 2017 Dec;14(6):573-580. doi: 10.1080/15412555.2017.1365120. Epub 2017 Sep 11. COPD. 2017. PMID: 28891722
Cited by
-
Suboptimal Peak Inspiratory Flow and Critical Inhalation Errors are Associated with Higher COPD-Related Healthcare Costs.Int J Chron Obstruct Pulmon Dis. 2022 Sep 25;17:2401-2415. doi: 10.2147/COPD.S380736. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 36185173 Free PMC article.
-
A Generative Adversarial Network (GAN) Technique for Internet of Medical Things Data.Sensors (Basel). 2021 May 27;21(11):3726. doi: 10.3390/s21113726. Sensors (Basel). 2021. PMID: 34071944 Free PMC article.
-
Physiological predictors Of peak inspiRatory flow using Observed lung function resultS (POROS): evaluation at discharge among patients hospitalized for a COPD exacerbation.Int J Chron Obstruct Pulmon Dis. 2018 Dec 13;13:3937-3946. doi: 10.2147/COPD.S174371. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30587952 Free PMC article.
-
Correct use and ease-of-use of placebo ELLIPTA dry-powder inhaler in adult patients with chronic obstructive pulmonary disease.PLoS One. 2022 Aug 15;17(8):e0273170. doi: 10.1371/journal.pone.0273170. eCollection 2022. PLoS One. 2022. PMID: 35969632 Free PMC article. Clinical Trial.
-
A randomized controlled trial of long-acting muscarinic antagonist and long-acting β2 agonist fixed-dose combinations in patients with chronic obstructive pulmonary disease.BMC Pulm Med. 2021 Jan 13;21(1):26. doi: 10.1186/s12890-021-01403-y. BMC Pulm Med. 2021. PMID: 33441146 Free PMC article. Clinical Trial.
References
-
- Lavorini F. Inhaled drug delivery in the hands of the patient. J Aerosol Med Pulm Drug Deliv. 2014;27(6):414–418. - PubMed
-
- Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371. - PubMed
-
- Lavorini F, Fontana GA. Inhaler technique and patient’s preference for dry powder inhaler devices. Expert Opin Drug Deliv. 2014;11(1):1–3. - PubMed
-
- Anderson P. Patient preference for and satisfaction with inhaler devices. European Res Rev. 2005;14(96):109–116.
-
- Berger BA. Assessing and interviewing patients for meaningful behavioral change: Part 1. Case Manager. 2004;15(5):46–50. quiz 51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

