Pharmacokinetics, distribution and anti-tumor efficacy of liposomal mitoxantrone modified with a luteinizing hormone-releasing hormone receptor-specific peptide
- PMID: 29520138
- PMCID: PMC5833774
- DOI: 10.2147/IJN.S150512
Pharmacokinetics, distribution and anti-tumor efficacy of liposomal mitoxantrone modified with a luteinizing hormone-releasing hormone receptor-specific peptide
Abstract
Background: A previous study developed a novel luteinizing hormone-releasing hormone (LHRH) receptor-targeted liposome. The aim of this study was to further assess the pharmacokinetics, biodistribution, and anti-tumor efficacy of LHRH receptor-targeted liposomes loaded with the anticancer drug mitoxantrone (MTO).
Methods: Plasma and tissue distribution profiles of LHRH receptor-targeted MTO-loaded liposomes (LHRH-MTO-LIPs) were quantified in healthy mice or a xenograft tumor nude mouse model of MCF-7 breast cancer, and were compared with non-targeted liposomes and a free-drug solution.
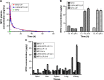
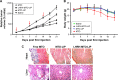
Results: The LHRH-MTO-LIPs demonstrated a superior pharmacokinetic profile relative to free MTO. The first target site of accumulation is the kidney, followed by the liver, and then the tumor; maximal tumor accumulation occurs at 4 h post-administration. Moreover, the LHRH-MTO-LIPs exhibited enhanced inhibition of MCF-7 breast cancer cell growth in vivo compared with non-targeted MTO-loaded liposomes (MTO-LIPs) and free MTO.
Conclusion: The novel LHRH receptor-targeted liposome may become a viable platform for the future targeted treatment of cancer.
Keywords: gonadorelin; liposome; luteinizing hormone-releasing hormone receptor; mitoxantrone; tumor targeting.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Luteinizing hormone-releasing hormone receptor-mediated delivery of mitoxantrone using LHRH analogs modified with PEGylated liposomes.Int J Nanomedicine. 2010 Sep 20;5:697-705. doi: 10.2147/ijn.s12129. Int J Nanomedicine. 2010. PMID: 20957221 Free PMC article.
-
Plasma membrane targeting by short chain sphingolipids inserted in liposomes improves anti-tumor activity of mitoxantrone in an orthotopic breast carcinoma xenograft model.Eur J Pharm Biopharm. 2015 Aug;94:207-19. doi: 10.1016/j.ejpb.2015.05.003. Epub 2015 May 14. Eur J Pharm Biopharm. 2015. PMID: 25982691
-
Design of multifunctional magnetic iron oxide nanoparticles/mitoxantrone-loaded liposomes for both magnetic resonance imaging and targeted cancer therapy.Int J Nanomedicine. 2014 Aug 22;9:4055-66. doi: 10.2147/IJN.S61880. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25187709 Free PMC article.
-
AEZS-108 : a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors.Expert Opin Investig Drugs. 2012 Jun;21(6):891-9. doi: 10.1517/13543784.2012.685128. Expert Opin Investig Drugs. 2012. PMID: 22577891 Review.
-
Targeting of cytotoxic luteinizing hormone-releasing hormone analogs to breast, ovarian, endometrial, and prostate cancers.Biol Reprod. 2005 Nov;73(5):851-9. doi: 10.1095/biolreprod.105.043489. Epub 2005 Jul 20. Biol Reprod. 2005. PMID: 16033997 Review.
Cited by
-
Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies.J Hematol Oncol. 2022 Sep 12;15(1):132. doi: 10.1186/s13045-022-01320-5. J Hematol Oncol. 2022. PMID: 36096856 Free PMC article. Review.
-
Nanocarrier-Mediated Chemo-Immunotherapy Arrested Cancer Progression and Induced Tumor Dormancy in Desmoplastic Melanoma.ACS Nano. 2018 Aug 28;12(8):7812-7825. doi: 10.1021/acsnano.8b01890. Epub 2018 Jul 23. ACS Nano. 2018. PMID: 30016071 Free PMC article.
-
Pharmacokinetics of protein and peptide conjugates.Drug Metab Pharmacokinet. 2019 Feb;34(1):42-54. doi: 10.1016/j.dmpk.2018.11.001. Epub 2018 Nov 22. Drug Metab Pharmacokinet. 2019. PMID: 30573392 Free PMC article. Review.
-
In silico study of chikungunya polymerase, a potential target for inhibitors.Virusdisease. 2019 Sep;30(3):394-402. doi: 10.1007/s13337-019-00547-0. Epub 2019 Oct 26. Virusdisease. 2019. PMID: 31803807 Free PMC article.
-
Integrin αvβ3 and LHRH Receptor Double Directed Nano-Analogue Effective Against Ovarian Cancer in Mice Model.Int J Nanomedicine. 2024 Mar 28;19:3071-3086. doi: 10.2147/IJN.S442921. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38562611 Free PMC article.
References
-
- Graham SM, Carlisle R, Choi JJ, et al. Inertial cavitation to non-invasively trigger and monitor intratumoral release of drug from intravenously delivered liposomes. J Control Release. 2014;178:101–107. - PubMed
-
- Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. - PubMed
-
- Zununi Vahed S, Salehi R, Davaran S, Sharifi S. Liposome-based drug co-delivery systems in cancer cells. Mater Sci Eng C Mater Biol Appl. 2017;71:1327–1341. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

