The antidepressant action of 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid is mediated by phosphorylation of histone deacetylase 5
- PMID: 29520168
- PMCID: PMC5840074
- DOI: 10.4196/kjpp.2018.22.2.155
The antidepressant action of 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid is mediated by phosphorylation of histone deacetylase 5
Abstract
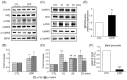
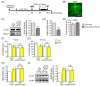
3-(2-Carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP), a competitive N-methyl-D-aspartate (NMDA) receptor antagonist, produces rapid antidepressant-like effects in animal models of depression. However, the molecular mechanisms underlying these behavioral actions remain unknown. Here, we demonstrate that CPP rapidly stimulates histone deacetylase (HDAC) 5 phosphorylation and nuclear export in rat hippocampal neurons. These effects are accompanied by calcium/calmodulin kinase II (CaMKII) and protein kinase D (PKD) phosphorylation. Behavioral experiments revealed that viral-mediated hippocampal knockdown of HDAC5 blocked the antidepressant effects of CPP in stressed animals. Taken together, our results imply that CPP acts via HDAC5 and suggest that HDAC5 is a common regulator contributing to the antidepressant actions of NMDA receptor antagonists such as CPP.
Keywords: Depression; Hippocampus; Histone deacetylase 5; NMDA receptor antagonist; Phosphorylation.
Figures


Similar articles
-
Ketamine produces antidepressant-like effects through phosphorylation-dependent nuclear export of histone deacetylase 5 (HDAC5) in rats.Proc Natl Acad Sci U S A. 2015 Dec 22;112(51):15755-60. doi: 10.1073/pnas.1513913112. Epub 2015 Dec 8. Proc Natl Acad Sci U S A. 2015. PMID: 26647181 Free PMC article.
-
Concentration-jump experiments with NMDA antagonists in mouse cultured hippocampal neurons.J Neurophysiol. 1990 Jun;63(6):1373-84. doi: 10.1152/jn.1990.63.6.1373. J Neurophysiol. 1990. PMID: 1972740
-
Erythropoietin and carbamylated erythropoietin promote histone deacetylase 5 phosphorylation and nuclear export in rat hippocampal neurons.Biochem Biophys Res Commun. 2016 Jan 29;470(1):220-225. doi: 10.1016/j.bbrc.2016.01.039. Epub 2016 Jan 9. Biochem Biophys Res Commun. 2016. PMID: 26777998
-
Action of 3-((+/-)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP): a new and highly potent antagonist of N-methyl-D-aspartate receptors in the hippocampus.Brain Res. 1986 Sep 10;382(1):174-7. doi: 10.1016/0006-8993(86)90128-9. Brain Res. 1986. PMID: 2876750
-
Contribution of AMPA and NMDA receptors to excitatory responses in the inferior colliculus.Hear Res. 2002 Jun;168(1-2):35-42. doi: 10.1016/s0378-5955(02)00372-6. Hear Res. 2002. PMID: 12117507 Review.
Cited by
-
(2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions.Proc Natl Acad Sci U S A. 2019 Mar 26;116(13):6441-6450. doi: 10.1073/pnas.1819540116. Epub 2019 Mar 13. Proc Natl Acad Sci U S A. 2019. PMID: 30867285 Free PMC article.
References
-
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry. 2008;64:880–883. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

