Plexin-Semaphorin Signaling Modifies Neuromuscular Defects in a Drosophila Model of Peripheral Neuropathy
- PMID: 29520219
- PMCID: PMC5827687
- DOI: 10.3389/fnmol.2018.00055
Plexin-Semaphorin Signaling Modifies Neuromuscular Defects in a Drosophila Model of Peripheral Neuropathy
Abstract
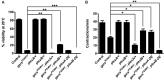
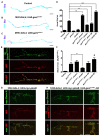
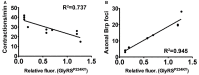
Dominant mutations in GARS, encoding the ubiquitous enzyme glycyl-tRNA synthetase (GlyRS), cause peripheral nerve degeneration and Charcot-Marie-Tooth disease type 2D (CMT2D). This genetic disorder exemplifies a recurring paradigm in neurodegeneration, in which mutations in essential genes cause selective degeneration of the nervous system. Recent evidence suggests that the mechanism underlying CMT2D involves extracellular neomorphic binding of mutant GlyRS to neuronally-expressed proteins. Consistent with this, our previous studies indicate a non-cell autonomous mechanism, whereby mutant GlyRS is secreted and interacts with the neuromuscular junction (NMJ). In this Drosophila model for CMT2D, we have previously shown that mutant gars expression decreases viability and larval motor function, and causes a concurrent build-up of mutant GlyRS at the larval neuromuscular presynapse. Here, we report additional phenotypes that closely mimic the axonal branching defects of Drosophila plexin transmembrane receptor mutants, implying interference of plexin signaling in gars mutants. Individual dosage reduction of two Drosophila Plexins, plexin A (plexA) and B (plexB) enhances and represses the viability and larval motor defects caused by mutant GlyRS, respectively. However, we find plexB levels, but not plexA levels, modify mutant GlyRS association with the presynaptic membrane. Furthermore, increasing availability of the plexB ligand, Semaphorin-2a (Sema2a), alleviates the pathology and the build-up of mutant GlyRS, suggesting competition for plexB binding may be occurring between these two ligands. This toxic gain-of-function and subversion of neurodevelopmental processes indicate that signaling pathways governing axonal guidance could be integral to neuropathology and may underlie the non-cell autonomous CMT2D mechanism.
Keywords: Charcot-Marie-Tooth disease type 2D (CMT2D); GARS; aminoacyl-tRNA synthetase (ARS); axonal guidance; distal spinal muscular atrophy type V (dSMA-V); glycyl-tRNA synthetase; neurodevelopment; neuromuscular disease.
Figures


Similar articles
-
Drosophila Models for Charcot-Marie-Tooth Neuropathy Related to Aminoacyl-tRNA Synthetases.Genes (Basel). 2021 Sep 27;12(10):1519. doi: 10.3390/genes12101519. Genes (Basel). 2021. PMID: 34680913 Free PMC article. Review.
-
Altered Sensory Neuron Development in CMT2D Mice Is Site-Specific and Linked to Increased GlyRS Levels.Front Cell Neurosci. 2020 Aug 11;14:232. doi: 10.3389/fncel.2020.00232. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32848623 Free PMC article.
-
Dominant, toxic gain-of-function mutations in gars lead to non-cell autonomous neuropathology.Hum Mol Genet. 2015 Aug 1;24(15):4397-406. doi: 10.1093/hmg/ddv176. Epub 2015 May 13. Hum Mol Genet. 2015. PMID: 25972375 Free PMC article.
-
CMT2D neuropathy is linked to the neomorphic binding activity of glycyl-tRNA synthetase.Nature. 2015 Oct 29;526(7575):710-4. doi: 10.1038/nature15510. Epub 2015 Oct 21. Nature. 2015. PMID: 26503042 Free PMC article.
-
Associations between Neurological Diseases and Mutations in the Human Glycyl-tRNA Synthetase.Biochemistry (Mosc). 2021 Jan;86(Suppl 1):S12-S23. doi: 10.1134/S0006297921140029. Biochemistry (Mosc). 2021. PMID: 33827397 Free PMC article. Review.
Cited by
-
Motor defects in a Drosophila model for spinal muscular atrophy result from SMN depletion during early neurogenesis.PLoS Genet. 2022 Jul 25;18(7):e1010325. doi: 10.1371/journal.pgen.1010325. eCollection 2022 Jul. PLoS Genet. 2022. PMID: 35877682 Free PMC article.
-
Boosting BDNF in muscle rescues impaired axonal transport in a mouse model of DI-CMTC peripheral neuropathy.bioRxiv [Preprint]. 2024 Mar 11:2023.04.09.536152. doi: 10.1101/2023.04.09.536152. bioRxiv. 2024. Update in: Neurobiol Dis. 2024 Jun 1;195:106501. doi: 10.1016/j.nbd.2024.106501. PMID: 38559020 Free PMC article. Updated. Preprint.
-
Transcriptional dysregulation by a nucleus-localized aminoacyl-tRNA synthetase associated with Charcot-Marie-Tooth neuropathy.Nat Commun. 2019 Nov 6;10(1):5045. doi: 10.1038/s41467-019-12909-9. Nat Commun. 2019. PMID: 31695036 Free PMC article.
-
Drosophila Models for Charcot-Marie-Tooth Neuropathy Related to Aminoacyl-tRNA Synthetases.Genes (Basel). 2021 Sep 27;12(10):1519. doi: 10.3390/genes12101519. Genes (Basel). 2021. PMID: 34680913 Free PMC article. Review.
-
CMT disease severity correlates with mutation-induced open conformation of histidyl-tRNA synthetase, not aminoacylation loss, in patient cells.Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19440-19448. doi: 10.1073/pnas.1908288116. Epub 2019 Sep 9. Proc Natl Acad Sci U S A. 2019. PMID: 31501329 Free PMC article.
References
-
- Achilli F., Bros-Facer V., Williams H. P., Banks G. T., AlQatari M., Chia R., et al. . (2009). An ENU-induced mutation in mouse glycyl-tRNA synthetase (GARS) causes peripheral sensory and motor phenotypes creating a model of Charcot-Marie-Tooth type 2D peripheral neuropathy. Dis. Model. Mech. 2, 359–373. 10.1242/dmm.002527 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

