Convenience versus Biological Significance: Are PMA-Differentiated THP-1 Cells a Reliable Substitute for Blood-Derived Macrophages When Studying in Vitro Polarization?
- PMID: 29520230
- PMCID: PMC5826964
- DOI: 10.3389/fphar.2018.00071
Convenience versus Biological Significance: Are PMA-Differentiated THP-1 Cells a Reliable Substitute for Blood-Derived Macrophages When Studying in Vitro Polarization?
Abstract
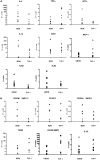
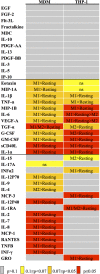
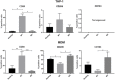
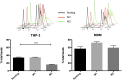
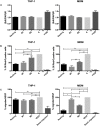
Human peripheral-blood monocytes are used as an established in vitro system for generating macrophages. For several reasons, monocytic cell lines such as THP-1 have been considered as a possible alternative. In view of their distinct developmental origins and phenotypic attributes, we set out to assess the extent to which human monocyte-derived macrophages (MDMs) and phorbol-12-myristate-13-acetate (PMA)-differentiated THP-1 cells were overlapping across a variety of responses to activating stimuli. Resting (M0) macrophages were polarized toward M1 or M2 phenotypes by 48-h incubation with LPS (1 μg/ml) and IFN-γ (10 ng/ml) or with IL-4 (20 ng/ml) and IL-13 (5 ng/ml), respectively. At the end of stimulation, MDMs displayed more pronounced changes in marker gene expression than THP-1. Upon assaying an array of 41 cytokines, chemokines and growth factors in conditioned media (CM) using the Luminex technology, secretion of 29 out of the 41 proteins was affected by polarized activation. While in 12 of them THP-1 and MDM showed comparable trends, for the remaining 17 proteins their responses to activating stimuli did markedly differ. Quantitative comparison for selected analytes confirmed this pattern. In terms of phenotypic activation markers, measured by flow cytometry, M1 response was similar but the established MDM M2 marker CD163 was undetectable in THP-1 cells. In a beads-based assay, MDM activation did not induce significant changes, whereas M2 activation of THP-1 decreased phagocytic activity compared to M0 and M1. In further biological activity tests, both MDM and THP-1 CM failed to affect proliferation of mouse myogenic progenitors, whereas they both reduced adipogenic differentiation of mouse fibro-adipogenic progenitor cells (M2 to a lesser extent than M1 and M0). Finally, migration of human umbilical vein endothelial cells was enhanced by CM irrespective of cell type and activation state except for M0 CM from MDMs. In summary, PMA-differentiated THP-1 macrophages did not entirely reproduce the response spectrum of primary MDMs to activating stimuli. We suggest that THP-1 be regarded as a simplified model of human macrophages when investigating relatively straightforward biological processes, such as polarization and its functional implications, but not as an alternative source in more comprehensive immunopharmacology and drug screening programs.
Keywords: THP-1 macrophages; human macrophages; macrophage activation; migration; phagocytosis; phenotype; progenitor cells.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

