Expression of Proinflammatory Cytokines Is Upregulated in the Hypothalamic Paraventricular Nucleus of Dahl Salt-Sensitive Hypertensive Rats
- PMID: 29520237
- PMCID: PMC5826963
- DOI: 10.3389/fphys.2018.00104
Expression of Proinflammatory Cytokines Is Upregulated in the Hypothalamic Paraventricular Nucleus of Dahl Salt-Sensitive Hypertensive Rats
Abstract
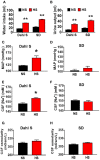
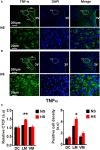
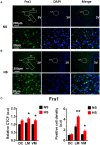
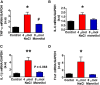
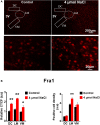
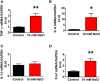
Accumulating evidence indicates that inflammation is implicated in hypertension. However, the role of brain proinflammatory cytokines (PICs) in salt sensitive hypertension remains to be determined. Thus, the objective of this study was to test the hypothesis that high salt (HS) diet increases PICs expression in the paraventricular nucleus (PVN) and leads to PVN neuronal activation. Eight-week-old male Dahl salt sensitive (Dahl S) rats, and age and sex matched normal Sprague Dawley (SD) rats were divided into two groups and fed with either a HS (4% NaCl) or normal salt (NS, 0.4% NaCl) diet for 5 consecutive weeks. HS diet induced hypertension and significantly increased cerebrospinal fluid (CSF) sodium concentration ([Na+]) in Dahl S rats, but not in normal SD rats. In addition, HS diet intake triggered increases in mRNA levels and immunoreactivities of PVN PICs including TNF-α, IL-6, and IL-1β, as well as Fra1, a chronic marker of neuronal activation, in Dahl S rats, but not in SD rats. Next, we investigated whether this increase in the expression of PVN PICs and Fra1 was induced by increased CSF [Na+]. Adult male SD rats were intracerebroventricular (ICV) infused with 8 μl of either hypertonic salt (4 μmol NaCl), mannitol (8 μmol, as osmolarity control), or isotonic salt (0.9% NaCl as vehicle control). Three hours following the ICV infusion, rats were euthanized and their PVN PICs expression was measured. The results showed that central administration of hypertonic saline in SD rats significantly increased the expression of PICs including TNF-α, IL-6, and IL-1β, as well as neuronal activation marker Fra1, compared to isotonic NaCl controls and osmolarity controls. Finally, we tested whether the increase in PICs expression occurred in neurons. Incubation of hypothalamic neurons with 10 mM NaCl in a culture medium for 6 h elicited significant increases in TNF-α, IL-6, and Fra1 mRNA levels. These observations, coupled with the important role of PICs in modulating neuronal activity and stimulating vasopressin release, suggest that HS intake induces an inflammatory state in the PVN, which, may in turn, augments sympathetic nerve activity and vasopressin secretion, contributing to the development of salt sensitive hypertension.
Keywords: cerebrospinal fluid; high salt diet; hypertension; paraventricular nucleus; proinflammatory cytokines.
Figures




References
-
- Bardgett M. E., Holbein W. W., Herrera-Rosales M., Toney G. M. (2014). Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-alpha. Hypertension 63, 527–534. 10.1161/HYPERTENSIONAHA.113.02429 - DOI - PMC - PubMed
-
- Biancardi V. C., Campos R. R., Stern J. E. (2010). Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J. Comp. Neurol. 518, 567–585. 10.1002/cne.22256 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

