Extracellular Vesicles Released from Mycobacterium tuberculosis-Infected Neutrophils Promote Macrophage Autophagy and Decrease Intracellular Mycobacterial Survival
- PMID: 29520273
- PMCID: PMC5827556
- DOI: 10.3389/fimmu.2018.00272
Extracellular Vesicles Released from Mycobacterium tuberculosis-Infected Neutrophils Promote Macrophage Autophagy and Decrease Intracellular Mycobacterial Survival
Abstract
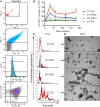
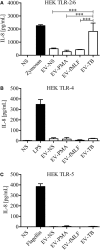
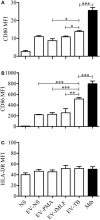
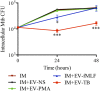
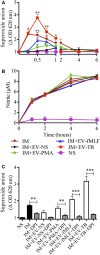
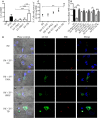
Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis (Mtb). In the lungs, macrophages and neutrophils are the first immune cells that have contact with the infecting mycobacteria. Neutrophils are phagocytic cells that kill microorganisms through several mechanisms, which include the lytic enzymes and antimicrobial peptides that are found in their lysosomes, and the production of reactive oxygen species. Neutrophils also release extracellular vesicles (EVs) (100-1,000 nm in diameter) to the extracellular milieu; these EVs consist of a lipid bilayer surrounding a hydrophilic core and participate in intercellular communication. We previously demonstrated that human neutrophils infected in vitro with Mtb H37Rv release EVs (EV-TB), but the effect of these EVs on other cells relevant for the control of Mtb infection, such as macrophages, has not been completely analyzed. In this study, we characterized the EVs produced by non-stimulated human neutrophils (EV-NS), and the EVs produced by neutrophils stimulated with an activator (PMA), a peptide derived from bacterial proteins (fMLF) or Mtb, and observed that the four EVs differed in their size. Ligands for toll-like receptor (TLR) 2/6 were detected in EV-TB, and these EVs favored a modest increase in the expression of the co-stimulatory molecules CD80, a higher expression of CD86, and the production of higher amounts of TNF-α and IL-6, and of lower amounts of TGF-β, in autologous human macrophages, compared with the other EVs. EV-TB reduced the amount of intracellular Mtb in macrophages, and increased superoxide anion production in these cells. TLR2/6 ligation and superoxide anion production are known inducers of autophagy; accordingly, we found that EV-TB induced higher expression of the autophagy-related marker LC3-II in macrophages, and the co-localization of LC3-II with Mtb inside infected macrophages. The intracellular mycobacterial load increased when autophagy was inhibited with wortmannin in these cells. In conclusion, our results demonstrate that neutrophils produce different EVs in response to diverse activators, and that EV-TB activate macrophages and promote the clearance of intracellular Mtb through early superoxide anion production and autophagy induction, which is a novel role for neutrophil-derived EVs in the immune response to Mtb.
Keywords: autophagy; extracellular vesicles; macrophage; neutrophils; tuberculosis.
Figures

References
-
- Tsao TC, Hong J, Huang C, Yang P, Liao SK, Chang KS. Increased TNF-alpha, IL-1 beta and IL-6 levels in the bronchoalveolar lavage fluid with the upregulation of their mRNA in macrophages lavaged from patients with active pulmonary tuberculosis. Tuber Lung Dis (1999) 79(5):279–85. 10.1054/tuld.1999.0215 - DOI - PubMed
-
- D’Avila H, Roque NR, Cardoso RM, Castro-Faria-Neto HC, Melo RC, Bozza PT. Neutrophils recruited to the site of Mycobacterium bovis BCG infection undergo apoptosis and modulate lipid body biogenesis and prostaglandin E production by macrophages. Cell Microbiol (2008) 10(12):2589–604. 10.1111/j.1462-5822.2008.01233.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

