Tolerogenic Dendritic Cells in Solid Organ Transplantation: Where Do We Stand?
- PMID: 29520275
- PMCID: PMC5827529
- DOI: 10.3389/fimmu.2018.00274
Tolerogenic Dendritic Cells in Solid Organ Transplantation: Where Do We Stand?
Abstract
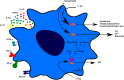
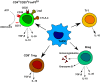
Over the past century, solid organ transplantation has been improved both at a surgical and postoperative level. However, despite the improvement in efficiency, safety, and survival, we are still far from obtaining full acceptance of all kinds of allograft in the absence of concomitant treatments. Today, transplanted patients are treated with immunosuppressive drugs (IS) to minimize immunological response in order to prevent graft rejection. Nevertheless, the lack of specificity of IS leads to an increase in the risk of cancer and infections. At this point, cell therapies have been shown as a novel promising resource to minimize the use of IS in transplantation. The main strength of cell therapy is the opportunity to generate allograft-specific tolerance, promoting in this way long-term allograft survival. Among several other regulatory cell types, tolerogenic monocyte-derived dendritic cells (Tol-MoDCs) appear to be an interesting candidate for cell therapy due to their ability to perform specific antigen presentation and to polarize immune response to immunotolerance. In this review, we describe the characteristics and the mechanisms of action of both human Tol-MoDCs and rodent tolerogenic bone marrow-derived DCs (Tol-BMDCs). Furthermore, studies performed in transplantation models in rodents and non-human primates corroborate the potential of Tol-BMDCs for immunoregulation. In consequence, Tol-MoDCs have been recently evaluated in sundry clinical trials in autoimmune diseases and shown to be safe. In addition to autoimmune diseases clinical trials, Tol-MoDC is currently used in the first phase I/II clinical trials in transplantation. Translation of Tol-MoDCs to clinical application in transplantation will also be discussed in this review.
Keywords: autologous tolerogenic dendritic cells; cell therapy; clinical trial; mechanisms; safety; transplantation.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

