Synthetic and semi-synthetic approaches to unprotected N-glycan oxazolines
- PMID: 29520306
- PMCID: PMC5827820
- DOI: 10.3762/bjoc.14.30
Synthetic and semi-synthetic approaches to unprotected N-glycan oxazolines
Abstract
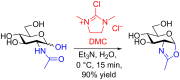
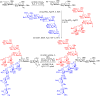
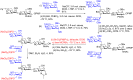
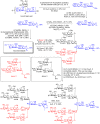
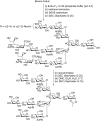
N-Glycan oxazolines have found widespread use as activated donor substrates for endo-β-N-acetylglucosaminidase (ENGase) enzymes, an important application that has correspondingly stimulated interest in their production, both by total synthesis and by semi-synthesis using oligosaccharides isolated from natural sources. Amongst the many synthetic approaches reported, the majority rely on the fabrication (either by total synthesis, or semi-synthesis from locust bean gum) of a key Manβ(1-4)GlcNAc disaccharide, which can then be elaborated at the 3- and 6-positions of the mannose unit using standard glycosylation chemistry. Early approaches subsequently relied on the Lewis acid catalysed conversion of peracetylated N-glycan oligosaccharides produced in this manner into their corresponding oxazolines, followed by global deprotection. However, a key breakthrough in the field has been the development by Shoda of 2-chloro-1,3-dimethylimidazolinium chloride (DMC), and related reagents, which can direct convert an oligosaccharide with a 2-acetamido sugar at the reducing terminus directly into the corresponding oxazoline in water. Therefore, oxazoline formation can now be achieved in water as the final step of any synthetic sequence, obviating the need for any further protecting group manipulations, and simplifying synthetic strategies. As an alternative to total synthesis, significant quantities of several structurally complicated N-glycans can be isolated from natural sources, such as egg yolks and soy bean flour. Enzymatic transformations of these materials, in concert with DMC-mediated oxazoline formation as a final step, allow access to a selection of N-glycan oxazoline structures both in larger quantities and in a more expedient fashion than is achievable by total synthesis.
Keywords: DMC, ENGase; N-glycans; glycosyl oxazolines; oligosaccharides.
Figures
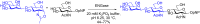



References
-
- Tews I, Terwisscha van Scheltinga A C, Perrakis A, Wilson K S, Dijkstra B W. J Am Chem Soc. 1997;119:7954–7959. doi: 10.1021/ja970674i. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
