Stimuli-responsive oligonucleotides in prodrug-based approaches for gene silencing
- PMID: 29520308
- PMCID: PMC5827813
- DOI: 10.3762/bjoc.14.32
Stimuli-responsive oligonucleotides in prodrug-based approaches for gene silencing
Abstract
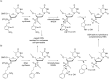
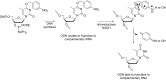
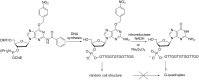
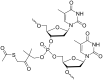
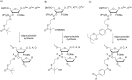
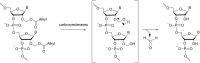
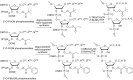
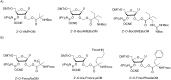
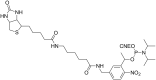
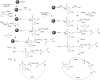
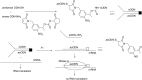
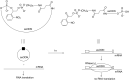
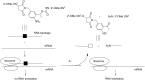
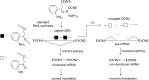
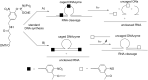
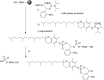
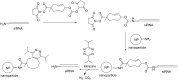
Oligonucleotides (ONs) have been envisaged for therapeutic applications for more than thirty years. However, their broad use requires overcoming several hurdles such as instability in biological fluids, low cell penetration, limited tissue distribution, and off-target effects. With this aim, many chemical modifications have been introduced into ONs definitively as a means of modifying and better improving their properties as gene silencing agents and some of them have been successful. Moreover, in the search for an alternative way to make efficient ON-based drugs, the general concept of prodrugs was applied to the oligonucleotide field. A prodrug is defined as a compound that undergoes transformations in vivo to yield the parent active drug under different stimuli. The interest in stimuli-responsive ONs for gene silencing functions has been notable in recent years. The ON prodrug strategies usually help to overcome limitations of natural ONs due to their low metabolic stability and poor delivery. Nevertheless, compared to permanent ON modifications, transient modifications in prodrugs offer the opportunity to regulate ON activity as a function of stimuli acting as switches. Generally, the ON prodrug is not active until it is triggered to release an unmodified ON. However, as it will be described in some examples, the opposite effect can be sought. This review examines ON modifications in response to various stimuli. These stimuli may be internal or external to the cell, chemical (glutathione), biochemical (enzymes), or physical (heat, light). For each stimulus, the discussion has been separated into sections corresponding to the site of the modification in the nucleotide: the internucleosidic phosphate, the nucleobase, the sugar or the extremities of ONs. Moreover, the review provides a current and detailed account of stimuli-responsive ONs with the main goal of gene silencing. However, for some stimuli-responsive ONs reported in this review, no application for controlling gene expression has been shown, but a certain potential in this field could be demonstrated. Additionally, other applications in different domains have been mentioned to extend the interest in such molecules.
Keywords: enzymolabile group; light-responsive group; oligonucleotide prodrugs; reduction-responsive; stimuli-responsive nucleic acids; thermolytic prodrugs.
Figures
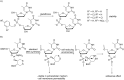
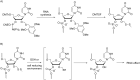
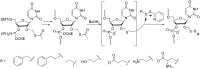
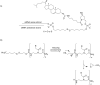

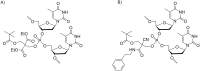



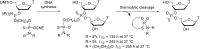

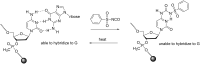

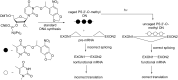


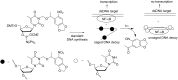
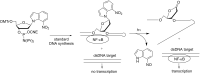
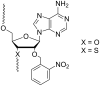
References
-
- Müller S. Molecules. 2017;22:No. 789. doi: 10.3390/molecules22050789. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
