Carbohydrate inhibitors of cholera toxin
- PMID: 29520310
- PMCID: PMC5827775
- DOI: 10.3762/bjoc.14.34
Carbohydrate inhibitors of cholera toxin
Abstract
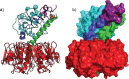
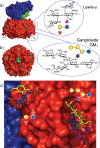
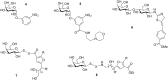
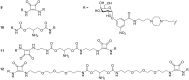
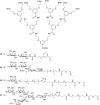
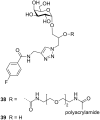
Cholera is a diarrheal disease caused by a protein toxin released by Vibrio cholera in the host's intestine. The toxin enters intestinal epithelial cells after binding to specific carbohydrates on the cell surface. Over recent years, considerable effort has been invested in developing inhibitors of toxin adhesion that mimic the carbohydrate ligand, with particular emphasis on exploiting the multivalency of the toxin to enhance activity. In this review we introduce the structural features of the toxin that have guided the design of diverse inhibitors and summarise recent developments in the field.
Keywords: carbohydrate; cholera; multivalency; toxin.
Figures

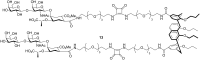


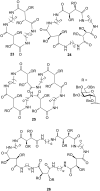

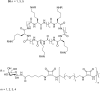
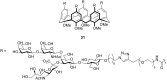




Similar articles
-
Modifications of cholera toxin subunit B binding to human large intestinal epithelium. An immunohistochemical study.Microb Pathog. 2018 Nov;124:332-336. doi: 10.1016/j.micpath.2018.08.047. Epub 2018 Aug 24. Microb Pathog. 2018. PMID: 30145256
-
Tetra- versus Pentavalent Inhibitors of Cholera Toxin.ChemistryOpen. 2015 Aug;4(4):471-7. doi: 10.1002/open.201500006. Epub 2015 Mar 21. ChemistryOpen. 2015. PMID: 26478842 Free PMC article.
-
Novel GM1 ganglioside-like peptide mimics prevent the association of cholera toxin to human intestinal epithelial cells in vitro.Glycobiology. 2016 Jan;26(1):63-73. doi: 10.1093/glycob/cwv080. Epub 2015 Sep 24. Glycobiology. 2016. PMID: 26405107 Free PMC article.
-
Transport of bacterial toxins into target cells: pathways followed by cholera toxin and botulinum progenitor toxin.J Biochem. 2006 Aug;140(2):155-60. doi: 10.1093/jb/mvj161. J Biochem. 2006. PMID: 16954533 Review.
-
Modulation of Host-Microbe Metabolism by Cholera Toxin.Infect Immun. 2023 May 16;91(5):e0043522. doi: 10.1128/iai.00435-22. Epub 2023 Apr 6. Infect Immun. 2023. PMID: 37022166 Free PMC article. Review.
Cited by
-
A Combination of Structural, Genetic, Phenotypic and Enzymatic Analyses Reveals the Importance of a Predicted Fucosyltransferase to Protein O-Glycosylation in the Bacteroidetes.Biomolecules. 2021 Nov 30;11(12):1795. doi: 10.3390/biom11121795. Biomolecules. 2021. PMID: 34944439 Free PMC article.
-
Strong Inhibition of Cholera Toxin B Subunit by Affordable, Polymer-Based Multivalent Inhibitors.Bioconjug Chem. 2019 Mar 20;30(3):785-792. doi: 10.1021/acs.bioconjchem.8b00902. Epub 2019 Jan 24. Bioconjug Chem. 2019. PMID: 30629410 Free PMC article.
-
Double-Modified Glycopolymers from Thiolactones to Modulate Lectin Selectivity and Affinity.ACS Macro Lett. 2018 Dec 18;7(12):1498-1502. doi: 10.1021/acsmacrolett.8b00825. Epub 2018 Dec 6. ACS Macro Lett. 2018. PMID: 30662815 Free PMC article.
-
Targeting Multiple Binding Sites on Cholera Toxin B with Glycomimetic Polymers Promotes the Formation of Protein-Polymer Aggregates.Biomacromolecules. 2020 Dec 14;21(12):4878-4887. doi: 10.1021/acs.biomac.0c01122. Epub 2020 Oct 5. Biomacromolecules. 2020. PMID: 32960582 Free PMC article.
-
Sodium butyrate inhibits the expression of virulence factors in Vibrio cholerae by targeting ToxT protein.mSphere. 2025 May 27;10(5):e0082424. doi: 10.1128/msphere.00824-24. Epub 2025 Apr 22. mSphere. 2025. PMID: 40261078 Free PMC article.
References
-
- WHO Wkly Epidemiol Rec. 2016;38:433–440. - PubMed
-
- Available from: http://www.gideononline.com.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
