Continuous multistep synthesis of 2-(azidomethyl)oxazoles
- PMID: 29520312
- PMCID: PMC5827817
- DOI: 10.3762/bjoc.14.36
Continuous multistep synthesis of 2-(azidomethyl)oxazoles
Abstract
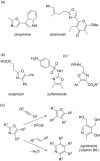
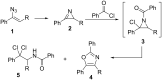
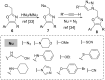
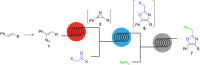
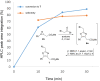
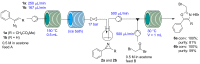
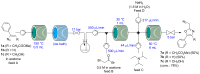
An efficient three-step protocol was developed to produce 2-(azidomethyl)oxazoles from vinyl azides in a continuous-flow process. The general synthetic strategy involves a thermolysis of vinyl azides to generate azirines, which react with bromoacetyl bromide to provide 2-(bromomethyl)oxazoles. The latter compounds are versatile building blocks for nucleophilic displacement reactions as demonstrated by their subsequent treatment with NaN3 in aqueous medium to give azido oxazoles in good selectivity. Process integration enabled the synthesis of this useful moiety in short overall residence times (7 to 9 min) and in good overall yields.
Keywords: azirines; continuous flow; heterocycles; oxazoles; process integration; vinyl azides.
Figures
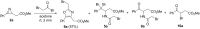
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
