EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection
- PMID: 29520645
- PMCID: PMC5990579
- DOI: 10.1007/s40264-018-0647-1
EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection
Abstract
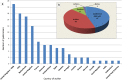
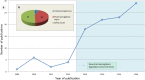
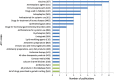
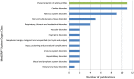
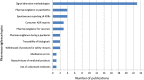
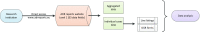
The analysis of safety data from spontaneous reporting systems has a proven value for the detection and analysis of the risks of medicines following their placement on the market and use in medical practice. EudraVigilance is the pharmacovigilance database to manage the collection and analysis of suspected adverse reactions to medicines authorised in the European Economic Area. EudraVigilance first operated in December 2001, with access to the database being governed by the EudraVigilance access policy. We performed a literature search including data up to December 2016 to demonstrate how the data from EudraVigilance has been used in scientific publications. We describe the results, including by type of publication, research topics and drugs involved. In 50% of the publications, the data are used to describe safety issues, in 44% to analyse methodologies used in pharmacovigilance activities and in 6% to support clinical perspectives. We also outline a description of the use of the database by the European Union regulatory network. Driven by the full implementation of the 2010 pharmacovigilance legislation, EudraVigilance has undergone further enhancements together with a major revision of its access policy, taking into account the use of the new individual case safety report standard developed by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use and the International Organization for Standardization. The aim of the broadened access is to facilitate more effective safety monitoring of authorised medicines, to make more data available for research and to provide better access to information on suspected adverse reactions for healthcare professionals and patients. In November 2017, the new full functionalities of EudraVigilance were launched, including the extensive web access to data on suspected adverse drug reactions and the possibilities for academic research institutions to request a more extensive dataset for the purposes of health research. The main objective of this article is to describe the new access to the database together with the opportunities that this new access can bring for research. It is intended to promote an appropriate use of the data to support the safe and effective use of medicines.
Conflict of interest statement
Funding
No sources of funding were received for the preparation of this review.
Conflict of interest
Rodrigo Postigo, Sabine Brosch, Jim Slattery, Xavier Kurz, Gianmario Candore, Francois Domergue and Peter Arlett are employees of the European Medicines Agency. Jean-Michel Dogné is a member of the Pharmacovigilance Risk Assessment Committee, as well as an employee of the Federal Agency for Medicines and Health Products of Belgium. Anja van Haren is an employee of the Medicines Evaluation Board of the Netherlands and she acts as a co-chair of the EudraVigilance Expert Working Group.
Disclaimer
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the agencies or organisations with which the authors are affiliated.
Figures
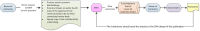
Similar articles
-
The Impact of Mandatory Reporting of Non-Serious Safety Reports to EudraVigilance on the Detection of Adverse Reactions.Drug Saf. 2022 Jan;45(1):83-95. doi: 10.1007/s40264-021-01137-0. Epub 2021 Dec 8. Drug Saf. 2022. PMID: 34881404 Free PMC article.
-
Safety monitoring of Influenza A/H1N1 pandemic vaccines in EudraVigilance.Vaccine. 2011 Jun 10;29(26):4378-87. doi: 10.1016/j.vaccine.2011.04.005. Epub 2011 Apr 16. Vaccine. 2011. PMID: 21501644
-
Strengthening and rationalizing pharmacovigilance in the EU: where is Europe heading to? A review of the new EU legislation on pharmacovigilance.Drug Saf. 2011 Mar 1;34(3):187-97. doi: 10.2165/11586620-000000000-00000. Drug Saf. 2011. PMID: 21332243 Review.
-
Spontaneous reports of vaccination errors in the European regulatory database EudraVigilance: A descriptive study.Vaccine. 2018 Dec 18;36(52):7956-7964. doi: 10.1016/j.vaccine.2018.11.003. Epub 2018 Nov 8. Vaccine. 2018. PMID: 30416019
-
The Importance of Direct Patient Reporting of Adverse Drug Reactions in the Safety Monitoring Process.Int J Environ Res Public Health. 2021 Dec 31;19(1):413. doi: 10.3390/ijerph19010413. Int J Environ Res Public Health. 2021. PMID: 35010673 Free PMC article. Review.
Cited by
-
Analysis of pharmacovigilance databases for spontaneous reports of adverse drug reactions related to substandard and falsified medical products: A descriptive study.Front Pharmacol. 2022 Sep 6;13:964399. doi: 10.3389/fphar.2022.964399. eCollection 2022. Front Pharmacol. 2022. PMID: 36147337 Free PMC article.
-
Developing Causality and Severity Assessment Frameworks for Food Safety Signals Using Social Media Reviews: A Technical Report Based on Data From an Urban Indian Suburb.Cureus. 2024 Jul 12;16(7):e64426. doi: 10.7759/cureus.64426. eCollection 2024 Jul. Cureus. 2024. PMID: 39130955 Free PMC article.
-
Development of a Pipeline for Adverse Drug Reaction Identification in Clinical Notes: Word Embedding Models and String Matching.JMIR Med Inform. 2022 Jan 25;10(1):e31063. doi: 10.2196/31063. JMIR Med Inform. 2022. PMID: 35076407 Free PMC article.
-
Seriousness and outcomes of reported adverse drug reactions in old and new antiseizure medications: a pharmacovigilance study using EudraVigilance database.Front Pharmacol. 2024 Jul 24;15:1411134. doi: 10.3389/fphar.2024.1411134. eCollection 2024. Front Pharmacol. 2024. PMID: 39119609 Free PMC article.
-
A Real-World Study on the Clinical Characteristics, Outcomes, and Relationship between Antibiotic Exposure and Clostridioides difficile Infection.Antibiotics (Basel). 2024 Feb 1;13(2):144. doi: 10.3390/antibiotics13020144. Antibiotics (Basel). 2024. PMID: 38391530 Free PMC article.
References
-
- European Medicines Agency. EudraVigilance. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/gen.... Accessed 31 Jan 2018.
-
- European Commission. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. https://ec.europa.eu/health/documents/eudralex/vol-1_en. Accessed 31 Jan 2018.
-
- European Commission. Regulation (EC) No. 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. https://ec.europa.eu/health/documents/eudralex/vol-1_en. Accessed 31 Jan 2018.
-
- European Commission. Commission Implementing Regulation (EU) No. 520/2012 of 19 June 2012 on the performance of pharmacovigilance activities provided for in Regulation (EC) No. 726/2004 of the European Parliament and of the Council and Directive 2001/83/EC of the European Parliament and of the Council. https://ec.europa.eu/health/documents/eudralex/vol-1_en. Accessed 31 Jan 2018.
-
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). http://www.ich.org/home.html. Accessed 31 Jan 2018.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

