A Mechanism-Based Population Pharmacokinetic Analysis Assessing the Feasibility of Efavirenz Dose Reduction to 400 mg in Pregnant Women
- PMID: 29520730
- PMCID: PMC6182466
- DOI: 10.1007/s40262-018-0642-9
A Mechanism-Based Population Pharmacokinetic Analysis Assessing the Feasibility of Efavirenz Dose Reduction to 400 mg in Pregnant Women
Abstract
Background: Reducing the dose of efavirenz can improve safety, reduce costs, and increase access for patients with HIV infection. According to the World Health Organization, a similar dosing strategy for all patient populations is desirable for universal roll-out; however, it remains unknown whether the 400 mg daily dose is adequate during pregnancy.
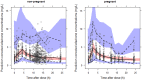
Methods: We developed a mechanistic population pharmacokinetic model using pooled data from women included in seven studies (1968 samples, 774 collected during pregnancy). Total and free efavirenz exposure (AUC24 and C12) were predicted for 400 (reduced) and 600 mg (standard) doses in both pregnant and non-pregnant women.
Results: Using a 400 mg dose, the median efavirenz total AUC24 and C12 during the third trimester of pregnancy were 91 and 87% of values among non-pregnant women, respectively. Furthermore, the median free efavirenz C12 and AUC24 were predicted to increase during pregnancy by 11 and 15%, respectively.
Conclusions: It was predicted that reduced-dose efavirenz provides adequate exposure during pregnancy. These findings warrant prospective confirmation.
Conflict of interest statement
Stein Schalkwijk, Rob ter Heine, Angela C. Colbers, Alwin D.R. Huitema, Paolo Denti, Kelly E. Dooley, Edmund Capparelli, Brookie M. Best, Tim R. Cressey, Rick Greupink, Frans G.M. Russel, Mark Mirochnick, and David M. Burger declare that they have no conflicts of interest.
Figures



Similar articles
-
Evaluation of universal versus genotype-guided efavirenz dose reduction in pregnant women using population pharmacokinetic modelling.J Antimicrob Chemother. 2018 Jan 1;73(1):165-172. doi: 10.1093/jac/dkx334. J Antimicrob Chemother. 2018. PMID: 29029267 Free PMC article.
-
Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis coinfection.J Infect Dis. 2015 Jan 15;211(2):197-205. doi: 10.1093/infdis/jiu429. Epub 2014 Jul 31. J Infect Dis. 2015. PMID: 25081933 Free PMC article.
-
Dosage Optimization of Efavirenz Based on a Population Pharmacokinetic-Pharmacogenetic Model of HIV-infected Patients in Thailand.Clin Ther. 2020 Jul;42(7):1234-1245. doi: 10.1016/j.clinthera.2020.04.013. Epub 2020 May 22. Clin Ther. 2020. PMID: 32451120
-
Efavirenz pharmacokinetics during the third trimester of pregnancy and postpartum.J Acquir Immune Defic Syndr. 2012 Mar 1;59(3):245-52. doi: 10.1097/QAI.0b013e31823ff052. J Acquir Immune Defic Syndr. 2012. PMID: 22083071 Free PMC article. Clinical Trial.
-
Efavirenz--still first-line king?Expert Opin Drug Metab Toxicol. 2008 Jul;4(7):965-72. doi: 10.1517/17425255.4.7.965. Expert Opin Drug Metab Toxicol. 2008. PMID: 18624683 Free PMC article. Review.
Cited by
-
Development of visual predictive checks accounting for multimodal parameter distributions in mixture models.J Pharmacokinet Pharmacodyn. 2019 Jun;46(3):241-250. doi: 10.1007/s10928-019-09632-9. Epub 2019 Apr 9. J Pharmacokinet Pharmacodyn. 2019. PMID: 30968312 Free PMC article.
-
Safety and Efficacy of Antiviral Drugs and Vaccines in Pregnant Women: Insights from Physiologically Based Pharmacokinetic Modeling and Integration of Viral Infection Dynamics.Vaccines (Basel). 2024 Jul 16;12(7):782. doi: 10.3390/vaccines12070782. Vaccines (Basel). 2024. PMID: 39066420 Free PMC article. Review.
-
Liver injury associated with drug intake during pregnancy.World J Hepatol. 2021 Jul 27;13(7):747-762. doi: 10.4254/wjh.v13.i7.747. World J Hepatol. 2021. PMID: 34367496 Free PMC article. Review.
-
Total and Free Blood and Plasma Concentration Changes in Pregnancy for Medicines Highly Bound to Plasma Proteins: Application of Physiologically Based Pharmacokinetic Modelling to Understand the Impact on Efficacy.Pharmaceutics. 2023 Oct 13;15(10):2455. doi: 10.3390/pharmaceutics15102455. Pharmaceutics. 2023. PMID: 37896215 Free PMC article.
References
-
- UNAIDS. Fact Sheet 2017. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_e....
-
- WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach – second edition. 2016 [updated June 2016. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. - PubMed
-
- WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Recommendations for a public health approach. 2010. Available at: www.who.int. - PubMed
-
- US FDA. Sustiva: prescribing information. 2011 Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
-
- DHHS. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2016 [updated 14 Jul 2016]. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

