Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics
- PMID: 29520731
- PMCID: PMC6132501
- DOI: 10.1007/s40262-018-0644-7
Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics
Abstract
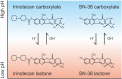
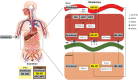
Since its clinical introduction in 1998, the topoisomerase I inhibitor irinotecan has been widely used in the treatment of solid tumors, including colorectal, pancreatic, and lung cancer. Irinotecan therapy is characterized by several dose-limiting toxicities and large interindividual pharmacokinetic variability. Irinotecan has a highly complex metabolism, including hydrolyzation by carboxylesterases to its active metabolite SN-38, which is 100- to 1000-fold more active compared with irinotecan itself. Several phase I and II enzymes, including cytochrome P450 (CYP) 3A4 and uridine diphosphate glucuronosyltransferase (UGT) 1A, are involved in the formation of inactive metabolites, making its metabolism prone to environmental and genetic influences. Genetic variants in the DNA of these enzymes and transporters could predict a part of the drug-related toxicity and efficacy of treatment, which has been shown in retrospective and prospective trials and meta-analyses. Patient characteristics, lifestyle and comedication also influence irinotecan pharmacokinetics. Other factors, including dietary restriction, are currently being studied. Meanwhile, a more tailored approach to prevent excessive toxicity and optimize efficacy is warranted. This review provides an updated overview on today's literature on irinotecan pharmacokinetics, pharmacodynamics, and pharmacogenetics.
Conflict of interest statement
Femke M. de Man, Andrew K.L. Goey, Ron H.N. van Schaik, Ron H.J. Mathijssen, and Sander Bins declare that they have no conflicts of interest.
Figures
Similar articles
-
Pharmacokinetic and Pharmacogenetic Markers of Irinotecan Toxicity.Curr Med Chem. 2019;26(12):2085-2107. doi: 10.2174/0929867325666180622141101. Curr Med Chem. 2019. PMID: 29932028 Review.
-
Mechanisms and emerging strategies for irinotecan-induced diarrhea.Eur J Pharmacol. 2024 Jul 5;974:176614. doi: 10.1016/j.ejphar.2024.176614. Epub 2024 Apr 25. Eur J Pharmacol. 2024. PMID: 38677535 Review.
-
Evaluation of pharmacogenomics and hepatic nuclear imaging-related covariates by population pharmacokinetic models of irinotecan and its metabolites.Eur J Clin Pharmacol. 2022 Jan;78(1):53-64. doi: 10.1007/s00228-021-03206-w. Epub 2021 Sep 4. Eur J Clin Pharmacol. 2022. PMID: 34480602
-
Prediction of irinotecan pharmacokinetics by use of cytochrome P450 3A4 phenotyping probes.J Natl Cancer Inst. 2004 Nov 3;96(21):1585-92. doi: 10.1093/jnci/djh298. J Natl Cancer Inst. 2004. PMID: 15523087
-
Effects of cyclosporin A on the pharmacokinetics and toxicities of irinotecan mediated by UGT1A1 in vitro and in vivo.Pharmazie. 2020 May 1;75(5):186-190. doi: 10.1691/ph.2020.0316. Pharmazie. 2020. PMID: 32393425
Cited by
-
Potential role of drug metabolizing enzymes in chemotherapy-induced gastrointestinal toxicity and hepatotoxicity.Expert Opin Drug Metab Toxicol. 2020 Nov;16(11):1109-1124. doi: 10.1080/17425255.2020.1815705. Epub 2020 Sep 2. Expert Opin Drug Metab Toxicol. 2020. PMID: 32841068 Free PMC article. Review.
-
On Broken Ne(c)ks and Broken DNA: The Role of Human NEKs in the DNA Damage Response.Cells. 2021 Feb 27;10(3):507. doi: 10.3390/cells10030507. Cells. 2021. PMID: 33673578 Free PMC article. Review.
-
Intraperitoneal irinotecan with concomitant FOLFOX and bevacizumab for patients with unresectable colorectal peritoneal metastases: protocol of the multicentre, open-label, phase II, INTERACT-II trial.BMJ Open. 2024 Jan 18;14(1):e077667. doi: 10.1136/bmjopen-2023-077667. BMJ Open. 2024. PMID: 38238055 Free PMC article.
-
Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties.Molecules. 2022 Aug 25;27(17):5436. doi: 10.3390/molecules27175436. Molecules. 2022. PMID: 36080203 Free PMC article. Review.
-
Liposomal irinotecan in metastatic pancreatic adenocarcinoma in Asian patients: Subgroup analysis of the NAPOLI-1 study.Cancer Sci. 2020 Feb;111(2):513-527. doi: 10.1111/cas.14264. Epub 2019 Dec 20. Cancer Sci. 2020. PMID: 31789476 Free PMC article. Clinical Trial.
References
-
- Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48(7):1722–1726. - PubMed
-
- Rivory LP, Robert J. Molecular, cellular, and clinical aspects of the pharmacology of 20(S)camptothecin and its derivatives. Pharmacol Ther. 1995;68(2):269–296. - PubMed
-
- Xie R, Mathijssen RH, Sparreboom A, Verweij J, Karlsson MO. Clinical pharmacokinetics of irinotecan and its metabolites: a population analysis. J Clin Oncol. 2002;20(15):3293–3301. - PubMed
-
- Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J Pharm Sci. 1992;81(7):676–684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

