Lessons From the Testosterone Trials
- PMID: 29522088
- PMCID: PMC6287281
- DOI: 10.1210/er.2017-00234
Lessons From the Testosterone Trials
Abstract
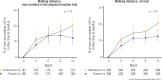
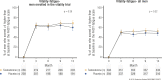
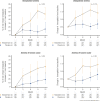
The Testosterone Trials (TTrials) were a coordinated set of seven placebo-controlled, double-blind trials in 788 men with a mean age of 72 years to determine the efficacy of increasing the testosterone levels of older men with low testosterone. Testosterone treatment increased the median testosterone level from unequivocally low at baseline to midnormal for young men after 3 months and maintained that level until month 12. In the Sexual Function Trial, testosterone increased sexual activity, sexual desire, and erectile function. In the Physical Function Trial, testosterone did not increase the distance walked in 6 minutes in men whose walk speed was slow; however, in all TTrial participants, testosterone did increase the distance walked. In the Vitality Trial, testosterone did not increase energy but slightly improved mood and depressive symptoms. In the Cognitive Function Trial, testosterone did not improve cognitive function. In the Anemia Trial, testosterone increased hemoglobin in both men who had anemia of a known cause and in men with unexplained anemia. In the Bone Trial, testosterone increased volumetric bone mineral density and the estimated strength of the spine and hip. In the Cardiovascular Trial, testosterone increased the coronary artery noncalcified plaque volume as assessed using computed tomographic angiography. Although testosterone was not associated with more cardiovascular or prostate adverse events than placebo, a trial of a much larger number of men for a much longer period would be necessary to determine whether testosterone increases cardiovascular or prostate risk.
Figures



References
-
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D; European Male Aging Study Group . Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737–2745. - PubMed
-
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86(2):724–731. - PubMed
-
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–598. - PubMed
-
- Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L, Lenrow DA, Holmes JH, Kapoor SC, Atkinson LE, Strom BL. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670–2677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

