Pacritinib vs Best Available Therapy, Including Ruxolitinib, in Patients With Myelofibrosis: A Randomized Clinical Trial
- PMID: 29522138
- PMCID: PMC5885169
- DOI: 10.1001/jamaoncol.2017.5818
Pacritinib vs Best Available Therapy, Including Ruxolitinib, in Patients With Myelofibrosis: A Randomized Clinical Trial
Abstract
Importance: Myelofibrosis is a hematologic malignancy characterized by splenomegaly and debilitating symptoms. Thrombocytopenia is a poor prognostic feature and limits use of Janus kinase 1 (JAK1)/Janus kinase 2 (JAK2) inhibitor ruxolitinib.
Objective: To compare the efficacy and safety of JAK2 inhibitor pacritinib with that of best available therapy (BAT), including ruxolitinib, in patients with myelofibrosis and thrombocytopenia.
Design, setting, and participants: For this phase 3 randomized international multicenter study-the PERSIST-2 study-of pacritinib vs BAT, 311 patients with myelofibrosis and platelet count 100 × 109/L or less were recruited for analysis. Crossover from BAT was allowed after week 24 or for progression of splenomegaly.
Interventions: Patients were randomized 1:1:1 to pacritinib 400 mg once daily, pacritinib 200 mg twice daily, or BAT.
Main outcomes and measures: Coprimary end points were rates of patients achieving 35% or more spleen volume reduction (SVR) and 50% or more reduction in total symptom score (TSS) at week 24. Efficacy analyses were performed on the intention-to-treat efficacy population, comprising all patients with a randomization date allowing for week 24 data.
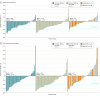
Results: Overall, 311 patients (mean [SD] age, 63.70 [9.08] years; 171 men [55%] and 140 women [45%]) were included in the study; 149 patients (48%) had prior ruxolitinib. The most common BAT was ruxolitinib (44 patients [45%]); 19 patients (19%) received watchful-waiting only. The intention-to-treat efficacy population included 75 patients randomized to pacritinib once daily; 74, pacritinib twice daily, and 72, BAT. Pacritinib (arms combined) was more effective than BAT for 35% or more SVR (27 patients [18%] vs 2 patients [3%]; P = .001) and had a nonsignificantly greater rate of 50% or more reduction in TSS (37 patients [25%] vs 10 patients [14%]; P = .08). Pacritinib twice daily led to significant improvements in both end points over BAT (≥35% SVR: 16 patients [22%] vs 2 patients [3%]; P = .001; ≥50% reduction in TSS: 24 patients [32%] vs 10 patients [14%]; P = .01). Clinical improvement in hemoglobin and reduction in transfusion burden were greatest with pacritinib twice daily. For pacritinib once daily, pacritinib twice daily, and BAT, the most common (>10%) grade 3 or 4 adverse events were thrombocytopenia (32 patients [31%], 34 patients [32%], 18 patients [18%]), and anemia (28 patients [27%], 23 patients [22%], 14 patients [14%]). In the pacritinib once daily, twice daily, and BAT arms, discontinuation owing to adverse events occurred in 15 patients (14%), 10 patients (9%), and 4 patients (4%).
Conclusions and relevance: In patients with myelofibrosis and thrombocytopenia, including those with prior anti-JAK therapy, pacritinib twice daily was more effective than BAT, including ruxolitinib, for reducing splenomegaly and symptoms.
Trial registration: clinicaltrials.gov Identifier: NCT02055781.
Conflict of interest statement
Figures

Comment in
-
Improved JAK Inhibition in Myelofibrosis-The Long Road Ahead.JAMA Oncol. 2018 May 1;4(5):659-660. doi: 10.1001/jamaoncol.2017.5802. JAMA Oncol. 2018. PMID: 29522105 No abstract available.
-
Pacritinib in Patients With Myelofibrosis.JAMA Oncol. 2018 Dec 1;4(12):1786-1787. doi: 10.1001/jamaoncol.2018.4830. JAMA Oncol. 2018. PMID: 30422169 No abstract available.
-
Pacritinib in Patients with Myelofibrosis-Reply.JAMA Oncol. 2018 Dec 1;4(12):1787. doi: 10.1001/jamaoncol.2018.4842. JAMA Oncol. 2018. PMID: 30422175 No abstract available.
References
-
- Cervantes F. How I treat myelofibrosis. Blood. 2014;124(17):2635-2642. - PubMed
-
- Gangat N, Caramazza D, Vaidya R, et al. . DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392-397. - PubMed
-
- Cervantes F, Dupriez B, Pereira A, et al. . New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895-2901. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

