Quality of Life During Treatment With Chemohormonal Therapy: Analysis of E3805 Chemohormonal Androgen Ablation Randomized Trial in Prostate Cancer
- PMID: 29522362
- PMCID: PMC5891128
- DOI: 10.1200/JCO.2017.75.3335
Quality of Life During Treatment With Chemohormonal Therapy: Analysis of E3805 Chemohormonal Androgen Ablation Randomized Trial in Prostate Cancer
Abstract
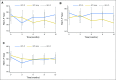
Purpose Chemohormonal therapy with docetaxel and androgen deprivation therapy (ADT+D) for metastatic hormone-sensitive prostate cancer improves overall survival as compared with androgen deprivation therapy (ADT) alone. We compared the quality of life (QOL) between patients with metastatic hormone-sensitive prostate cancer who were treated with ADT+D and those who were treated with ADT alone. Methods Men were randomly assigned to ADT+ D (six cycles) or to ADT alone. QOL was assessed by Functional Assessment of Cancer Therapy-Prostate (FACT-P), FACT-Taxane, Functional Assessment of Chronic Illness Therapy-Fatigue, and the Brief Pain Inventory at baseline and at 3, 6, 9, and 12 months. The Wilcoxon signed rank test was used to examine changes over time. Mixed-effect models compared the QOL between arms at each time point. Results Seven hundred ninety men were randomly assigned (ADT+D [n = 397] and ADT[ n = 393]) and completed FACT-P (90% at baseline, 86% at 3 months, 83% at 6 months, 78% at 9 months, and 77% at 12 months). ADT+D patients reported a statistically significant decline in FACT-P at 3 months ( P < .001) but FACT-P did not differ significantly between baseline and 12 months ( P = .38). ADT+D FACT-P scores were significantly lower at 3 months ( P = .02) but significantly higher at 12 months ( P = .04) when compared with ADT FACT-P scores. Differences did not exceed the minimal clinically important difference at any time point. ADT+D patients reported significantly lower Functional Assessment of Chronic Illness Therapy-Fatigue scores at 3 months than did ADT patients ( P < .001). Over time, both arms reported significantly poorer FACT-Taxane scores ( P < .001) when compared with baseline. Brief Pain Inventory scores were similar between arms. Conclusion Although ADT+D was associated with statistically worse QOL at 3 months, QOL was better at 12 months for ADT+D patients than for ADT patients. Both arms reported a similar minimally changed QOL over time, suggesting that ADT+D is not associated with a greater long-term negative impact on QOL.
Trial registration: ClinicalTrials.gov NCT00309985.
Figures

Comment in
-
Re: Quality of Life during Treatment with Chemohormonal Therapy: Analysis of E3805 Chemohormonal Androgen Ablation Randomized Trial in Prostate Cancer.J Urol. 2018 Nov;200(5):936. doi: 10.1016/j.juro.2018.08.022. Epub 2018 Aug 13. J Urol. 2018. PMID: 30360328 No abstract available.
References
-
- Ferlay J, Soerjomataram I, Ervik M, et al: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11, Lyon, France, International Agency for Research on Cancer, 2013.
-
- Key statistics for prostate cancer, in American Cancer Society 2017.
-
- Sweeney C, Chen YH, Liu G, et al: Long term efficacy and QOL data of chemohormonal therapy (C-HT) in low and high volume hormone naïve metastatic prostate cancer (PrCa): E3805 CHAARTED trial. European Society for Medical Oncology Annual Congress 2016, Copenhagen, Denmark, 2016 (abstr 720PD)
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180802/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- U10 CA180847/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180853/CA/NCI NIH HHS/United States
- UG1 CA189859/CA/NCI NIH HHS/United States
- UG1 CA189974/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

