Fropofol decreases force development in cardiac muscle
- PMID: 29522375
- PMCID: PMC6044060
- DOI: 10.1096/fj.201701442R
Fropofol decreases force development in cardiac muscle
Abstract
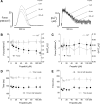
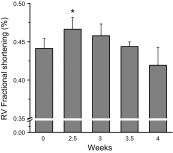
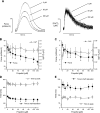
Supranormal contractile properties are frequently associated with cardiac diseases. Anesthetic agents, including propofol, can depress myocardial contraction. We tested the hypothesis that fropofol, a propofol derivative, reduces force development in cardiac muscles via inhibition of cross-bridge cycling and may therefore have therapeutic potential. Force and intracellular Ca2+ concentration ([Ca2+]i) transients of rat trabecular muscles were determined. Myofilament ATPase, actin-activated myosin ATPase, and velocity of actin filaments propelled by myosin were also measured. Fropofol dose dependently decreased force without altering [Ca2+]i in normal and pressure-induced hypertrophied-hypercontractile muscles. Similarly, fropofol depressed maximum Ca2+-activated force ( Fmax) and increased the [Ca2+]i required for 50% of Fmax (Ca50) at steady state without affecting the Hill coefficient in both intact and skinned cardiac fibers. The drug also depressed cardiac myofibrillar and actin-activated myosin ATPase activity. In vitro actin sliding velocity was significantly reduced when fropofol was introduced during rigor binding of cross-bridges. The data suggest that the depressing effects of fropofol on cardiac contractility are likely to be related to direct targeting of actomyosin interactions. From a clinical standpoint, these findings are particularly significant, given that fropofol is a nonanesthetic small molecule that decreases myocardial contractility specifically and thus may be useful in the treatment of hypercontractile cardiac disorders.-Ren, X., Schmidt, W., Huang, Y., Lu, H., Liu, W., Bu, W., Eckenhoff, R., Cammarato, A., Gao, W. D. Fropofol decreases force development in cardiac muscle.
Keywords: excitation contraction coupling; fropofol; in vitro motility; intracellular calcium; myofilament protein.
Conflict of interest statement
This research was supported, in part, by a Stimulating and Advancing Anesthesiology and Critical Care Medicine (ACCM) Research (StARR) Award from the Department of Anesthesiology and Critical Care Medicine (Johns Hopkins University School of Medicine; to W.D.G.); American Heart Association–Global Innovation Award (AHA-GIA) (17GRNT33670387; to W.D.G.); U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grants GM055867 and GM008076, and NIH National Institute of Neurological Disease and Stroke Grant NS080519 (to R.E.); NIH National Heart, Lung, and Blood Institute Grant T32HL007227-38 (to W.S.) and R01HL124091 (to A.C.); and AHA Grant 17POST33630159 (to W.S.). The authors declare no conflicts of interest.
Figures


Similar articles
-
Fropofol prevents disease progression in mice with hypertrophic cardiomyopathy.Cardiovasc Res. 2020 May 1;116(6):1175-1185. doi: 10.1093/cvr/cvz218. Cardiovasc Res. 2020. PMID: 31424496 Free PMC article.
-
Molecular mechanism of anesthetic-induced depression of myocardial contraction.FASEB J. 2016 Aug;30(8):2915-25. doi: 10.1096/fj.201600290RR. Epub 2016 May 11. FASEB J. 2016. PMID: 27170289 Free PMC article.
-
Anesthetic Agents Isoflurane and Propofol Decrease Maximal Ca2+-Activated Force and Thus Contractility in the Failing Myocardium.J Pharmacol Exp Ther. 2019 Dec;371(3):615-623. doi: 10.1124/jpet.119.259556. Epub 2019 Sep 12. J Pharmacol Exp Ther. 2019. PMID: 31515443 Free PMC article.
-
Regulation of contraction in striated muscle.Physiol Rev. 2000 Apr;80(2):853-924. doi: 10.1152/physrev.2000.80.2.853. Physiol Rev. 2000. PMID: 10747208 Review.
-
Regulation of cardiac contractile proteins. Correlations between physiology and biochemistry.Circ Res. 1984 Nov;55(5):565-74. doi: 10.1161/01.res.55.5.565. Circ Res. 1984. PMID: 6091939 Review.
Cited by
-
Fropofol prevents disease progression in mice with hypertrophic cardiomyopathy.Cardiovasc Res. 2020 May 1;116(6):1175-1185. doi: 10.1093/cvr/cvz218. Cardiovasc Res. 2020. PMID: 31424496 Free PMC article.
-
Human Resistin Induces Cardiac Dysfunction in Pulmonary Hypertension.J Am Heart Assoc. 2023 Mar 21;12(6):e027621. doi: 10.1161/JAHA.122.027621. Epub 2023 Mar 16. J Am Heart Assoc. 2023. PMID: 36927008 Free PMC article.
-
Right ventricular diastolic dysfunction and failure: a review.Heart Fail Rev. 2022 Jul;27(4):1077-1090. doi: 10.1007/s10741-021-10123-8. Epub 2021 May 19. Heart Fail Rev. 2022. PMID: 34013436 Review.
-
Time to Wake Up! The Ongoing Search for General Anesthetic Reversal Agents.Anesthesiology. 2024 Mar 1;140(3):610-627. doi: 10.1097/ALN.0000000000004846. Anesthesiology. 2024. PMID: 38349760 Free PMC article.
-
The actin 'A-triad's' role in contractile regulation in health and disease.J Physiol. 2020 Jul;598(14):2897-2908. doi: 10.1113/JP276741. Epub 2019 Mar 28. J Physiol. 2020. PMID: 30770548 Free PMC article. Review.
References
-
- Maron B. J., Bonow R. O., Cannon R. O., III, Leon M. B., Epstein S. E. (1987) Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (2). N. Engl. J. Med. 316, 844–852 - PubMed
-
- Maron B. J., Bonow R. O., Cannon R. O., III, Leon M. B., Epstein S. E. (1987) Hypertrophic cardiomyopathy: interrelations of clinical manifestations, pathophysiology, and therapy (1). N. Engl. J. Med. 316, 780–789 - PubMed
-
- Guinto P. J., Haim T. E., Dowell-Martino C. C., Sibinga N., Tardiff J. C. (2009) Temporal and mutation-specific alterations in Ca2+ homeostasis differentially determine the progression of cTnT-related cardiomyopathies in murine models. Am. J. Physiol. Heart Circ. Physiol. 297, H614–H626 - PMC - PubMed
-
- Schober T., Huke S., Venkataraman R., Gryshchenko O., Kryshtal D., Hwang H. S., Baudenbacher F. J., Knollmann B. C. (2012) Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ. Res. 111, 170–179 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

