Antifeedant Activities of Lignans from Stem Bark of Zanthoxylum armatum DC. against Tribolium castaneum
- PMID: 29522428
- PMCID: PMC6017925
- DOI: 10.3390/molecules23030617
Antifeedant Activities of Lignans from Stem Bark of Zanthoxylum armatum DC. against Tribolium castaneum
Abstract
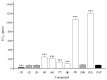
The speciation of a methanolic extract of Zanthoxylum armatum stem bark has enabled the isolation and characterization of 11 known lignans. Among them, five compounds (6, 8-11) are reported in this plant for the first time. All of the chemical structures were elucidated on the basis of NMR spectral analysis. Additionally, their antifeedant activities against Tribolium castaneum were evaluated scientifically. Among them, asarinin (1), with an EC50 of 25.64 ppm, exhibited a much stronger antifeedant activity than the positive control, toosendanin (EC50 = 71.69 ppm). Moreover, fargesin (2), horsfieldin (3), and magnolone (10), with EC50 values of 63.24, 68.39, and 78.37 ppm, showed almost the same antifeedant activity as the positive control. From the perspective of structure-effectiveness relationship, compounds with the chemical group of methylenedioxy exhibited higher antifeedant activities and have potential to be developed into novel antifeedants or potential lead compounds to protect food and crops in storage.
Keywords: Tribolium castaneum; Zanthoxylum armatum; antifeedant activity; chemical constituents; methylenedioxy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rajashekar Y., Shivanandappa T. A novel natural insecticide molecule for grain protection. Julius-Kühn-Archiv. 2010:910–915. doi: 10.5073/jka.2010.425.413.. - DOI
-
- Denell R., Gibbs R., Muzny D., Weinstock G.M., Attaway T., Bell S., Buhay C.J., Chandrabose M.N., Chavez D., Clerk-Blankenburg K.P., et al. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–955. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

