The Inside Story of Adenosine
- PMID: 29522447
- PMCID: PMC5877645
- DOI: 10.3390/ijms19030784
The Inside Story of Adenosine
Abstract
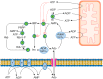
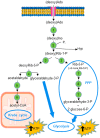
Several physiological functions of adenosine (Ado) appear to be mediated by four G protein-coupled Ado receptors. Ado is produced extracellularly from the catabolism of the excreted ATP, or intracellularly from AMP, and then released through its transporter. High level of intracellular Ado occurs only at low energy charge, as an intermediate of ATP breakdown, leading to hypoxanthine production. AMP, the direct precursor of Ado, is now considered as an important stress signal inside cell triggering metabolic regulation through activation of a specific AMP-dependent protein kinase. Intracellular Ado produced from AMP by allosterically regulated nucleotidases can be regarded as a stress signal as well. To study the receptor-independent effects of Ado, several experimental approaches have been proposed, such as inhibition or silencing of key enzymes of Ado metabolism, knockdown of Ado receptors in animals, the use of antagonists, or cell treatment with deoxyadenosine, which is substrate of the enzymes acting on Ado, but is unable to interact with Ado receptors. In this way, it was demonstrated that, among other functions, intracellular Ado modulates angiogenesis by regulating promoter methylation, induces hypothermia, promotes apoptosis in sympathetic neurons, and, in the case of oxygen and glucose deprivation, exerts a cytoprotective effect by replenishing the ATP pool.
Keywords: adenosine; adenosine deaminase; adenosine kinase; adenosine receptors; deoxyadenosine; energy repletion; transmethylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ipata P.L., Pesi R. Nucleoside recycling in the brain and the nucleosidome: A complex metabolic and molecular cross-talk between the extracellular nucleotide cascade system and the intracellular nucleoside salvage. Metabolomics. 2016;12:22. doi: 10.1007/s11306-015-0931-3. - DOI
-
- Ashby B., Holmsen H. Platelet AMP deaminase: Regulation by Mg–ATP2- and inorganic phosphate and inhibition by the transition state analog coformycin. J. Biol. Chem. 1983;258:3668–3672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

