Biological Activities of Stilbenoids
- PMID: 29522491
- PMCID: PMC5877653
- DOI: 10.3390/ijms19030792
Biological Activities of Stilbenoids
Abstract
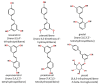
Stilbenoids are a group of naturally occurring phenolic compounds found in various plant species. They share a common backbone structure known as stilbene, but differ in the nature and position of substituents. Stilbenoids are classified as phytoalexins, which are antimicrobial compounds produced de novo in plants to protect against fungal infection and toxins. In this review, the biological effects of stilbenoids such as resveratrol, pterostilbene, gnetol and piceatannol are discussed. Stilbenoids exert various biological activities ranging from cardioprotection, neuroprotection, anti-diabetic properties, depigmentation, anti-inflammation, cancer prevention and treatment. The results presented cover a myriad of models, from cell culture to animal studies as well as clinical human trials. Although positive results were obtained in most cell culture and animal studies, further human studies are needed to substantiate beneficial effects of stilbenoids. Resveratrol remains the most widely studied stilbenoid. However, there is limited information regarding the potential of less common stilbenoids. Therefore, further research is warranted to evaluate the salutary effects of various stilbenoids.
Keywords: biological activities; cardioprotective; gnetol; pterostilbene; resveratrol; stilbenoid phenolics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Langcake P., Pryce R.J. The production of resveratrol by vitis vinifera and other members of the vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976;9:77–86. doi: 10.1016/0048-4059(76)90077-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical