Differential role of MyD88 and TRIF signaling in myeloid cells in the pathogenesis of autoimmune diabetes
- PMID: 29522531
- PMCID: PMC5844544
- DOI: 10.1371/journal.pone.0194048
Differential role of MyD88 and TRIF signaling in myeloid cells in the pathogenesis of autoimmune diabetes
Abstract
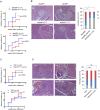
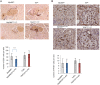
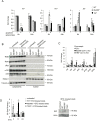
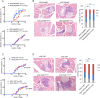
Type 1 diabetes (T1D) is caused by the autoimmune destruction of the insulin-producing pancreatic beta cells. While the role of adaptive immunity has been extensively studied, the role of innate immune responses and particularly of Toll- like Receptor (TLR) signaling in T1D remains poorly understood. Here we show that myeloid cell-specific MyD88 deficiency considerably protected mice from the development of streptozotocin (STZ)-induced diabetes. The protective effect of MyD88 deficiency correlated with increased expression of the immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO) in pancreatic lymph nodes from STZ-treated mice and in bone marrow-derived dendritic cells (BMDC) stimulated with apoptotic cells. Mice with myeloid cell specific TIR-domain-containing adapter-inducing interferon-β (TRIF) knockout showed a trend towards accelerated onset of STZ-induced diabetes, while TRIF deficiency resulted in reduced IDO expression in vivo and in vitro. Moreover, myeloid cell specific MyD88 deficiency delayed the onset of diabetes in Non-Obese Diabetic (NOD) mice, whereas TRIF deficiency had no effect. Taken together, these results identify MyD88 signaling in myeloid cells as a critical pathogenic factor in autoimmune diabetes, which is antagonized by TRIF-dependent responses. This differential function of MyD88 and TRIF depends at least in part on their opposite effects in regulating IDO expression in phagocytes exposed to apoptotic cells.
Conflict of interest statement
Figures

References
-
- Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10(7):501–13. Epub 2010/06/26. doi: 10.1038/nri2787. . - DOI - PubMed
-
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. Epub 2005/03/18. doi: 10.1146/annurev.immunol.23.021704.115643. . - DOI - PubMed
-
- Pearson JA, Wong FS, Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun. 2016;66:76–88. Epub 2015/09/26. doi: 10.1016/j.jaut.2015.08.019. ; PubMed Central PMCID: PMCPMC4765310. - DOI - PMC - PubMed
-
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–26. Epub 2007/12/19. doi: 10.1007/s00125-007-0886-7. . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

