Protein-Injection Machines in Bacteria
- PMID: 29522749
- PMCID: PMC5849082
- DOI: 10.1016/j.cell.2018.01.034
Protein-Injection Machines in Bacteria
Abstract
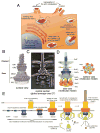
Many bacteria have evolved specialized nanomachines with the remarkable ability to inject multiple bacterially encoded effector proteins into eukaryotic or prokaryotic cells. Known as type III, type IV, and type VI secretion systems, these machines play a central role in the pathogenic or symbiotic interactions between multiple bacteria and their eukaryotic hosts, or in the establishment of bacterial communities in a diversity of environments. Here we focus on recent progress elucidating the structure and assembly pathways of these machines. As many of the interactions shaped by these machines are of medical importance, they provide an opportunity to develop novel therapeutic approaches to combat important human diseases.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
-
- Akeda Y, Galan JE. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. - PubMed
-
- Alouf J. Bacterial protein toxins: an overview. Methods Mol Biol. 2000;145:1–26. - PubMed
-
- Anantharajah A, Mingeot-Leclercq M, Van Bambeke F. Targeting the Type Three Secretion System in Pseudomonas aeruginosa. Trends Pharmacol Sci. 2016;37:734–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

