Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma
- PMID: 29522834
- PMCID: PMC5911193
- DOI: 10.1016/j.jconrel.2018.03.006
Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma
Abstract
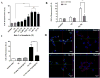
Glioblastomas (GBMs) are highly aggressive brain tumors with a very grim prognosis even after multi-modal therapeutic regimens. Conventional chemotherapeutic agents frequently lead to drug resistance and result in severe toxicities to non-cancerous tissues. Resveratrol (RES), a natural polyphenol with pleiotropic health benefits, has proven chemopreventive effects in all the stages of cancer including initiation, promotion and progression. However, the poor physico-chemical properties of RES severely limit its use as a free drug. In this study, RES was loaded into PEGylated liposomes (RES-L) to counter its drawbacks as a free drug. Since transferrin receptors (TfRs) are up-regulated in GBM, the liposome surface was modified with transferrin moieties (Tf-RES-L) to make them cancer cell-specific. The liposomal nanomedicines developed in this project were aimed at enhancing the physico-chemical properties of RES and exploiting the passive and active targeting capabilities of liposomes to effectively treat GBM. The RES-L were stable, had a good drug-loading capacity, prolonged drug-release in vitro and were easily scalable. Flow cytometry and confocal microscopy were used to study the association with, and internalization of, Tf-L into U-87 MG cells. The Tf-RES-Ls were significantly more cytotoxic and induced higher levels of apoptosis accompanied by activation of caspases 3/7 in GBM cells when compared to free RES or RES-L. The ability of RES to arrest cells in the S-phase of the cell cycle, and selectively induce production of reactive oxygen species in cancer cells were probably responsible for its cytotoxic effects. The therapeutic efficacy of RES formulations was evaluated in a subcutaneous xenograft mouse model of GBM. A tumor growth inhibition study and a modified survival study showed that Tf-RES-Ls were more effective than other treatments in their ability to inhibit tumor growth and improve survival in mice. Overall, the liposomal nanomedicines of RES developed in this project exhibited favorable in vitro and in vivo efficacies, which warrant their further investigation for the treatment of GBMs.
Keywords: Cancer; Drug delivery; Glioblastoma; Liposomes; Resveratrol; Targeting; Transferrin.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

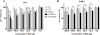



References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Friedmann-Morvinski D. Glioblastoma heterogeneity and cancer cell plasticity. Crit Rev Oncog. 2014;19:327–336. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

