Visual quality assessment of the liver graft by the transplanting surgeon predicts postreperfusion syndrome after liver transplantation: a retrospective cohort study
- PMID: 29523082
- PMCID: PMC5845208
- DOI: 10.1186/s12871-018-0493-9
Visual quality assessment of the liver graft by the transplanting surgeon predicts postreperfusion syndrome after liver transplantation: a retrospective cohort study
Abstract
Background: The discrepancy between demand and supply for liver transplants (LT) has led to an increased transplantation of organs from extended criteria donors (ECD).
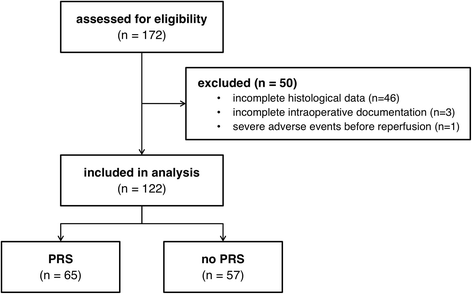
Methods: In this single center retrospective analysis of 122 cadaveric LT recipients, we investigated predictors of postreperfusion syndrome (PRS) including transplant liver quality categorized by both histological assessment of steatosis and subjective visual assessment by the transplanting surgeon using multivariable regression analysis. Furthermore, we describe the relevance of PRS during the intraoperative and postoperative course of LT recipients.
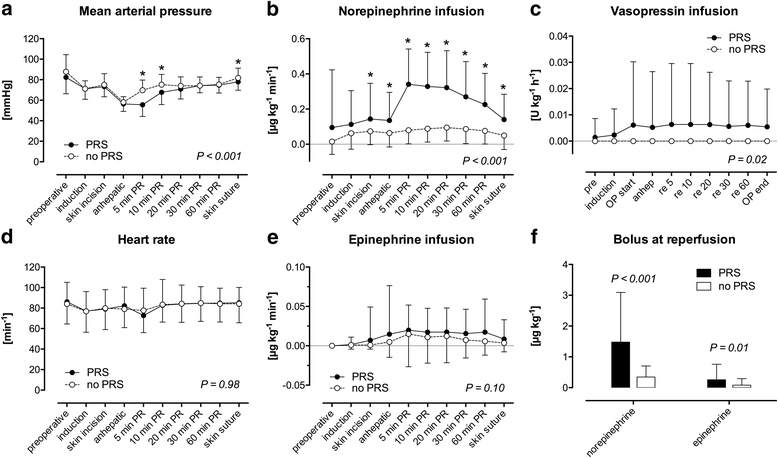
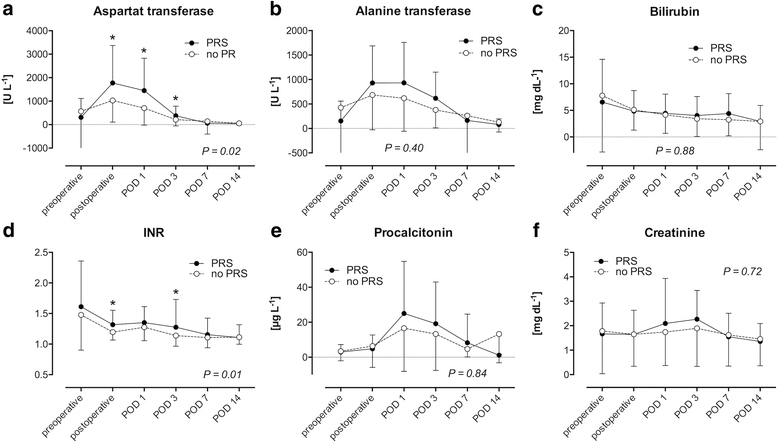
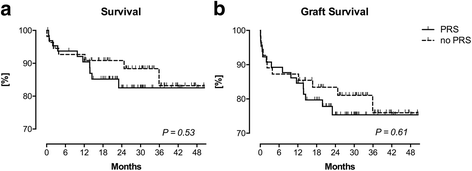
Results: 53.3% (n = 65) of the patients suffered from PRS. Risk factors for PRS were visually assessed organ quality of the liver grafts (acceptable: OR 12.2 [95% CI 2.43-61.59], P = 0.002; poor: OR 13.4 [95% CI 1.48-121.1], P = 0.02) as well as intraoperative norepinephrine dosage before reperfusion (OR 2.2 [95% CI 1.26-3.86] per 0.1 μg kg- 1 min- 1, P = 0.01). In contrast, histological assessment of the graft was not associated with PRS. LT recipients suffering from PRS were hemodynamically more instable after reperfusion compared to recipients not suffering from PRS. They had lower mean arterial pressures until the end of surgery (P < 0.001), received more epinephrine and norepinephrine before reperfusion (P = 0.02 and P < 0.001, respectively) as well as higher rates of continuous infusion of norepinephrine (P < 0.001) and vasopressin (P = 0.02) after reperfusion. Postoperative peak AST was significantly higher (P = 0.001) in LT recipients with PRS. LT recipients with intraoperative PRS had more postoperative adverse cardiac events (P = 0.05) and suffered more often from postoperative delirium (P = 0.04).
Conclusions: Patients receiving ECD liver grafts are especially prone to PRS. Anesthesiologists should keep these newly described risk factors in mind when preparing for reperfusion in patients receiving high-risk organs.
Keywords: Cold ischemia time; Hyponatremia; Steatosis.
Conflict of interest statement
Ethics approval and consent to participate
The local ethics committee (University Hospital RWTH Aachen, EK 291/13) approved the analysis and waived the requirement of informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Tranplants by Donor Type [https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/]. Accessed 10 May 2017.
-
- Annual Report 2015 [http://www.eurotransplant.org/cms/mediaobject.php?file=AR_ET_20153.pdf]. Accessed 8 May 2017.
-
- Schlitt HJ, Loss M, Scherer MN, Becker T, Jauch KW, Nashan B, Schmidt H, Settmacher U, Rogiers X, Neuhaus P, et al. Current developments in liver transplantation in Germany: MELD-based organ allocation and incentives for transplant centres. Z Gastroenterol. 2011;49(1):30–38. doi: 10.1055/s-0029-1245946. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

