MYC amplification in subtypes of breast cancers in African American women
- PMID: 29523126
- PMCID: PMC5845301
- DOI: 10.1186/s12885-018-4171-6
MYC amplification in subtypes of breast cancers in African American women
Abstract
Background: MYC overexpression is associated with poor prognosis in breast tumors (BCa). The objective of this study was to determine the prevalence of MYC amplification and associated markers in BCa tumors from African American (AA) women and determine the associations between MYC amplification and clinico-pathological characteristics.
Methods: We analyzed 70 cases of well characterized archival breast ductal carcinoma specimens from AA women for MYC oncogene amplification. Utilizing immune histochemical analysis estrogen receptor (ER), progesterone receptor (PR), and (HER2/neu), were assessed. Cases were Luminal A (ER or PR+, Ki-67 < 14%), Luminal B (ER or PR+, Ki-67 = > 14% or ER or PR+ HER2+), HER2 (ER-, PR-, HER2+), and Triple Negative (ER-, PR-, HER2-) with basal-like phenotype. The relationship between MYC amplification and prognostic clinico-pathological characteristics was determined using chi square and logistic regression modeling.
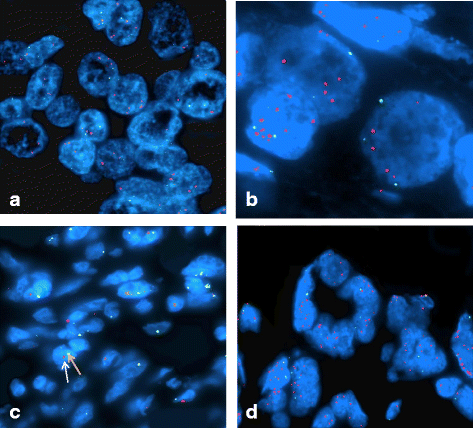
Results: Sixty-five (97%) of the tumors showed MYC gene amplification (MYC: CEP8 > 1). Statistically significant associations were found between MYC amplification and HER2-amplified BCa, and Luminal B subtypes of BCa (p < 0.0001), stage (p < 0.001), metastasis (p < 0.001), and positive lymph node status (p = 0.039). MYC amplification was associated with HER2 status (p = 0.01) and tumor size (p = 0.01). High MYC amplification was seen in grade III carcinomas (MYC: CEP8 = 2.42), pre-menopausal women (MYC: CEP8 = 2.49), PR-negative status (MYC: CEP8 = 2.42), and ER-positive status (MYC: CEP8 = 2.4).
Conclusions: HER2 positive BCas in AA women are likely to exhibit MYC amplification. High amplification ratios suggest that MYC drives HER2 amplification, especially in HER2 positive, Luminal B, and subtypes of BCa.
Keywords: Breast cancer subtypes; FISH; Gene amplification; MYC.
Conflict of interest statement
Ethics approval and consent to participate
This study was based on existing de-identified data and pathologic specimens. It was not deemed to be human subjects’ research, but determined to be of exempt status. Approval for this research protocol was given by the Howard University School of Medicine IRB, designated as reference number 15CMED-53.
Consent for publication
Subject consent was not required for this study. It is bound by the ruling of the US Health and Human Services Secretary’s Advisory Committee on Human Research Protections (SACHRP) under the Common Rule for exempt research and the Helsinki guidelines for establishment of IRB committees. IRB approval #15C MED 53 was obtained from the Howard University IRB for the study of de-identified materials.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Copeland G LA, Firth R, et al. Cancer in North America: 2008–2012. North American Association of Central Cancer Registries, Inc, Springfield, IL. June 2015.;Volume One: Combined Cancer Incidence for the United States, Canada and North America.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

