Study on the progression types of cancer in patients with breast cancer undergoing eribulin chemotherapy and tumor microenvironment
- PMID: 29523158
- PMCID: PMC5845371
- DOI: 10.1186/s12967-018-1443-5
Study on the progression types of cancer in patients with breast cancer undergoing eribulin chemotherapy and tumor microenvironment
Abstract
Background: Recently, the concepts of progression due to pre-existing lesions (PPL) and progression due to new metastasis (PNM) have been proposed to differentiate the progression types of treatment-resistant cancers. Previously, the differences between these two progression types did not affect the determination of treatment strategies since both PPL and PNM are classified as progressive disease based on the response evaluation criteria in solid tumors (RECIST) diagnostic criteria. On the other hand, tumor infiltrating lymphocytes (TILs) are effective when used as indicators for monitoring the immune tumor microenvironment (iTME) in the cancer host, and TILs play an important role as biomarkers in predicting prognosis and therapeutic effects. This study focused on the progression types of cancer in patients undergoing eribulin chemotherapy. In addition, the iTME in individuals with PPL and PNM was evaluated using TILs as a marker.
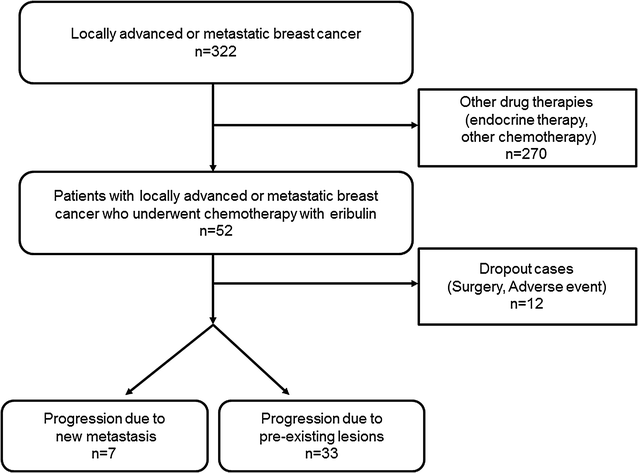
Methods: Of the 52 patients with locally advanced or metastatic breast cancer who underwent chemotherapy with eribulin, 40 remained in the study, and 12 patients were dropout cases. The antitumor effect was evaluated based on the RECIST criteria using version 1.1. TILs were defined as the infiltrating lymphocytes within tumor stroma and were expressed in proportion to the field investigated. In PPL cases, the high-TIL group was considered as type I and the low-TIL group was classified as type II. In PNM cases, the high-TIL group was considered as type III and the low-TIL group was classified as type IV.
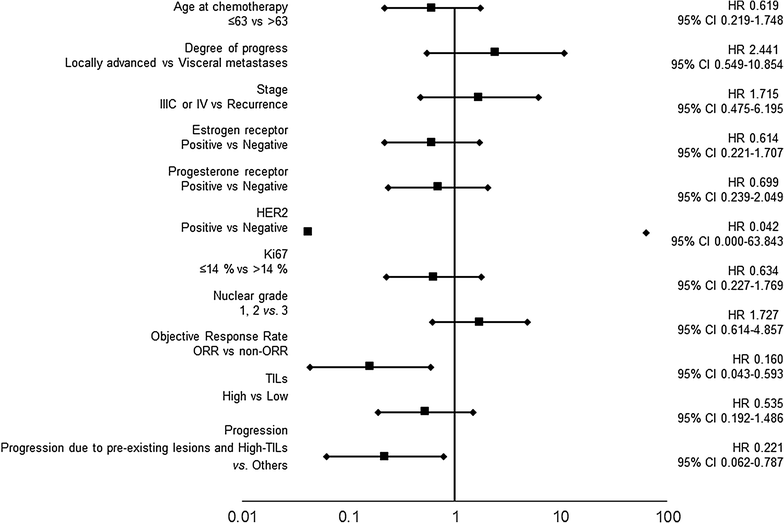
Results: In 19 cases, individuals with type I progression had significantly longer progression free survival and overall survival (OS) compared to those with type III progression (p = 0.040, p < 0.001, log-rank). Individuals with type I progression had significantly prolonged survival post progression compared to those with type II progression (p = 0.048, log-rank). A multivariate analysis that validate the effect of OS showed that these were independent factors of good prognosis (p = 0.003; hazard ratio [HR] = 0.065) (p = 0.006; HR = 0.105).
Conclusions: The effects of eribulin chemotherapy suggested that patients with progressive-type breast cancer that proliferates in a good iTME may have a good prognosis.
Keywords: Breast cancer; Eribulin; Progressive disease; Tumor microenvironment; Tumor-infiltrating lymphocytes.
Figures



Similar articles
-
Use of Tumor-infiltrating lymphocytes (TILs) to predict the treatment response to eribulin chemotherapy in breast cancer.PLoS One. 2017 Feb 6;12(2):e0170634. doi: 10.1371/journal.pone.0170634. eCollection 2017. PLoS One. 2017. PMID: 28166544 Free PMC article.
-
Prediction of survival after eribulin chemotherapy for breast cancer by absolute lymphocyte counts and progression types.World J Surg Oncol. 2021 Nov 15;19(1):324. doi: 10.1186/s12957-021-02441-w. World J Surg Oncol. 2021. PMID: 34775950 Free PMC article.
-
Eribulin Promotes Antitumor Immune Responses in Patients with Locally Advanced or Metastatic Breast Cancer.Anticancer Res. 2018 May;38(5):2929-2938. doi: 10.21873/anticanres.12541. Anticancer Res. 2018. PMID: 29715119
-
Eribulin mesylate: a novel halichondrin B analogue for the treatment of metastatic breast cancer.Am J Health Syst Pharm. 2012 May 1;69(9):745-55. doi: 10.2146/ajhp110237. Am J Health Syst Pharm. 2012. PMID: 22517020 Review.
-
Eribulin in advanced breast cancer: safety, efficacy and new perspectives.Future Oncol. 2017 Dec;13(30):2759-2769. doi: 10.2217/fon-2017-0283. Epub 2017 Sep 13. Future Oncol. 2017. PMID: 29219017 Review.
Cited by
-
Clinical verification of the relationship between smoking and the immune microenvironment of breast cancer.J Transl Med. 2019 Jan 7;17(1):13. doi: 10.1186/s12967-019-1773-y. J Transl Med. 2019. PMID: 30616624 Free PMC article.
-
N-Cadherin mRNA Levels in Peripheral Blood Could Be a Potential Indicator of New Metastases in Breast Cancer: A Pilot Study.Int J Mol Sci. 2020 Jan 14;21(2):511. doi: 10.3390/ijms21020511. Int J Mol Sci. 2020. PMID: 31947504 Free PMC article.
-
Clinical significance of plasma MMP-2 and MMP-9 levels as biomarkers for tumor expression in breast cancer patients in Egypt.Mol Biol Rep. 2020 Feb;47(2):1153-1160. doi: 10.1007/s11033-019-05216-5. Epub 2019 Dec 9. Mol Biol Rep. 2020. PMID: 31820313
-
Real-world efficacy and safety of eribulin in advanced and pretreated HER2-negative breast cancer in a Spanish comprehensive cancer center.BMC Pharmacol Toxicol. 2019 Nov 21;20(1):68. doi: 10.1186/s40360-019-0367-x. BMC Pharmacol Toxicol. 2019. PMID: 31753013 Free PMC article.
-
Tumour necrosis factor-alpha levels as predictor factor on clinical response of anthracycline-based neoadjuvant chemotherapy in locally advance breast cancer patients: experimental research.Ann Med Surg (Lond). 2023 Apr 3;85(4):807-811. doi: 10.1097/MS9.0000000000000424. eCollection 2023 Apr. Ann Med Surg (Lond). 2023. PMID: 37113871 Free PMC article.
References
-
- Litiere S, de Vries EG, Seymour L, Sargent D, Shankar L, Bogaerts J, Committee R The components of progression as explanatory variables for overall survival in the response evaluation criteria in solid tumours 1.1 database. Eur J Cancer. 2014;50(10):1847–1853. doi: 10.1016/j.ejca.2014.03.014. - DOI - PubMed
-
- Twelves C, Cortes J, Kaufman PA, Yelle L, Awada A, Binder TA, Olivo M, Song J, O’Shaughnessy JA, Jove M, et al. “New” metastases are associated with a poorer prognosis than growth of pre-existing metastases in patients with metastatic breast cancer treated with chemotherapy. Breast Cancer Res. 2015;17(1):150. doi: 10.1186/s13058-015-0657-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

