Dedicated mobile application for drug adverse reaction reporting by patients with relapsing remitting multiple sclerosis (Vigip-SEP study): study protocol for a randomized controlled trial
- PMID: 29523169
- PMCID: PMC5845183
- DOI: 10.1186/s13063-018-2560-4
Dedicated mobile application for drug adverse reaction reporting by patients with relapsing remitting multiple sclerosis (Vigip-SEP study): study protocol for a randomized controlled trial
Abstract
Background: The reporting of adverse drug reactions (ADR) by patients represents an interesting challenge in the field of pharmacovigilance, but the reporting system is not adequately implemented in France. In 2015, only 20 MS patients in France reported ADR due to first-line disease-modifying drugs (DMD), while more than 3000 patients were initiated on DMD. The aim of this study is to validate a proof-of-concept as to whether the use of a mobile application (App) increases ADR reporting among patients with relapsing-remitting multiple sclerosis (RR-MS) receiving DMD.
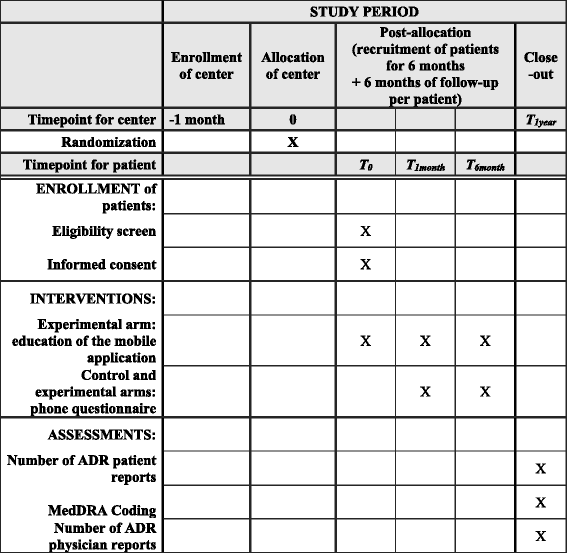
Methods/design: We designed a multi-centric, open cluster-randomized controlled trial, called the Vigip-SEP study (NCT03029897), using the App My eReport France® to report ADR to the appropriate authorities in E2B language, in accordance with European regulations. RR-MS patients who were initiated on, or switched, first-line DMD will be included. In the experimental arm, a neurologist will introduce the patient to the App to report ADR to the appropriate French authorities. In the control arm, the patient will be informed of the existence of the App but will not be introduced to its use and will then report ADR according to the usual reporting procedures. Primary assessment criteria are defined as the average number of ADR per patient and per center. We assume that the App will increase patient reporting by 10-fold. Therefore, we will require 24 centers (12 per arm: 6 MS academic expert centers, 3 general hospitals, 3 private practice neurologists), allowing for an expected enrollment of 180 patients (alpha risk 5%, power 90% and standard deviation 4%).
Discussion: Increasing patient reporting of ADR in a real-life setting is extremely important for therapeutic management of RR-MS, particularly for monitoring newly approved DMD to gain better knowledge of their safety profiles. To increase patient involvement, teaching patients to use tools, such as mobile applications, should be encouraged, and these tools should be tested rigorously.
Trial registration: ClinicalTrials.gov , ID: NCT03029897 . Registered on 20 January 2017.
Keywords: Adverse drug reaction; E-health; Mobile application; Multiple sclerosis; Patient reporting; Pharmacovigilance.
Conflict of interest statement
Authors' information
Not applicable
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Nord-Ouest III with approval number 2016–42. Informed consent will be obtained from all participants.
Consent for publication
The authors consent to publication of this article.
Competing interests
The funders had no involvement in the collection, management, analysis and interpretation of data. The authors and investigators declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Härmark L, van Hunsel F, Grundmark B. ADR Reporting by the general public: lessons learnt from the Dutch and Swedish Systems. Drug Saf. 2015;38(4):337–47. 10.1007/s40264-015-0264-1. Accessed 3 July 2018. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials