Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails
- PMID: 29523187
- PMCID: PMC5845228
- DOI: 10.1186/s13287-018-0794-4
Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails
Abstract
Background: The advent of human-induced pluripotent stem cells holds great promise for producing ample individualized hepatocytes. Although previous efforts have succeeded in generating hepatocytes from human pluripotent stem cells in vitro by viral-based expression of transcription factors and/or addition of growth factors during the differentiation process, the safety issue of viral transduction and high cost of cytokines would hinder the downstream applications. Recently, the use of small molecules has emerged as a powerful tool to induce cell fate transition for their superior stability, safety, cell permeability, and cost-effectiveness.
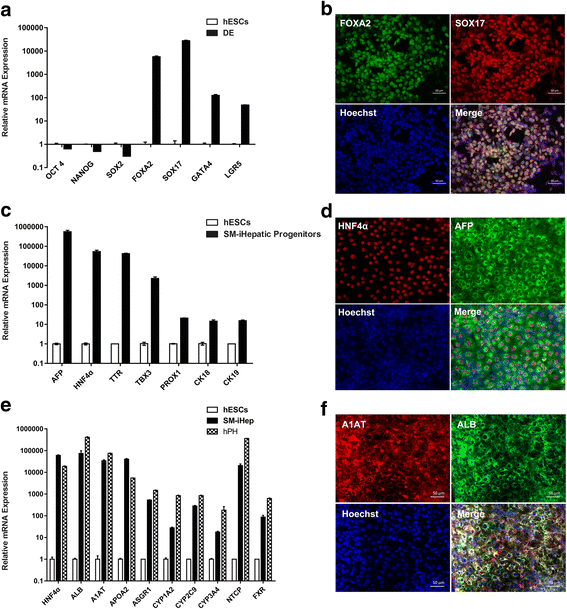
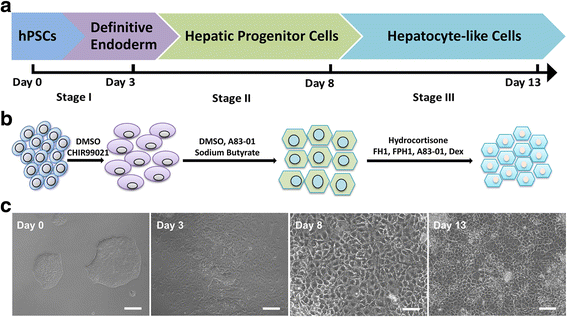
Methods: In the present study, we established a novel efficient hepatocyte differentiation strategy of human pluripotent stem cells with pure small-molecule cocktails. This method induced hepatocyte differentiation in a stepwise manner, including definitive endoderm differentiation, hepatic specification, and hepatocyte maturation within only 13 days.
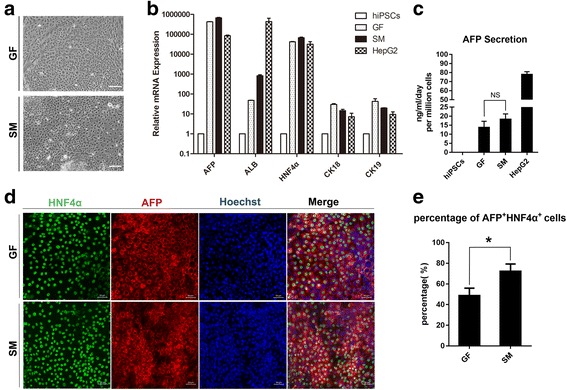
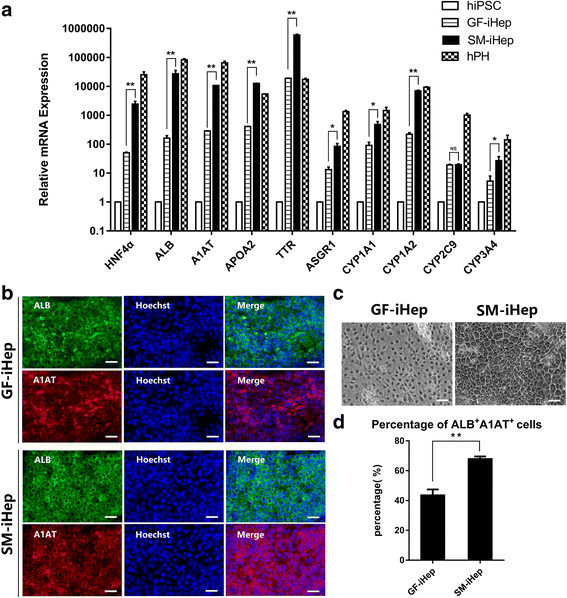
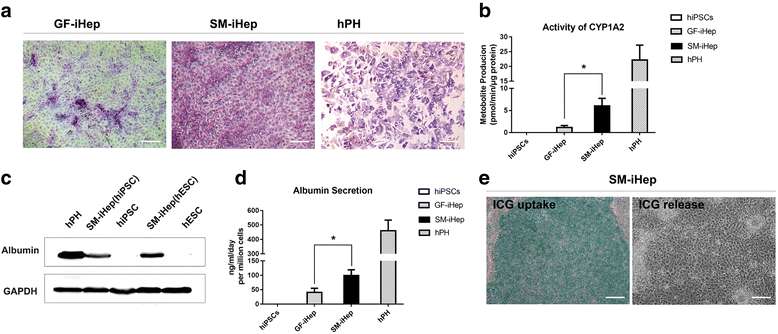
Results: The differentiated hepatic-like cells were morphologically similar to hepatocytes derived from growth factor-based methods and primary hepatocytes. These cells not only expressed specific hepatic markers at the transcriptional and protein levels, but also possessed main liver functions such as albumin production, glycogen storage, cytochrome P450 activity, and indocyanine green uptake and release.
Conclusions: Highly efficient and expedited hepatic differentiation from human pluripotent stem cells could be achieved by our present novel, pure, small-molecule cocktails strategy, which provides a cost-effective platform for in vitro studies of the molecular mechanisms of human liver development and holds significant potential for future clinical applications.
Keywords: Hepatocyte differentiation; Human pluripotent stem cells; Small molecules.
Conflict of interest statement
Ethics approval and consent to participate
Fresh liver tissues were obtained from a DCD donor for liver transplantation in the Third Affiliated Hospital of Sun Yat-sen University. The use of liver tissue samples for research purposes was approved by the Medical Ethics Committee of the Third Affiliated Hospital of SYSU, and all informed consent was obtained.
Consent for publication
All the authors have looked through the manuscript and approved the submission.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Retaining mTeSR1 Medium during Hepatic Differentiation Facilitates Hepatocyte-Like Cell Survival by Decreasing Apoptosis.Cell Physiol Biochem. 2018;51(4):1533-1543. doi: 10.1159/000495644. Epub 2018 Nov 29. Cell Physiol Biochem. 2018. PMID: 30497075
-
Isolation and expansion of human pluripotent stem cell-derived hepatic progenitor cells by growth factor defined serum-free culture conditions.Exp Cell Res. 2017 Mar 15;352(2):333-345. doi: 10.1016/j.yexcr.2017.02.022. Epub 2017 Feb 17. Exp Cell Res. 2017. PMID: 28215634
-
Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells.Stem Cell Reports. 2015 May 12;4(5):939-52. doi: 10.1016/j.stemcr.2015.04.001. Epub 2015 Apr 30. Stem Cell Reports. 2015. PMID: 25937370 Free PMC article.
-
Human hepatocytes derived from pluripotent stem cells: a promising cell model for drug hepatotoxicity screening.Arch Toxicol. 2016 Sep;90(9):2049-2061. doi: 10.1007/s00204-016-1756-1. Epub 2016 Jun 20. Arch Toxicol. 2016. PMID: 27325232 Review.
-
Human-Induced Pluripotent Stem Cell-Derived Hepatocytes and their Culturing Methods to Maintain Liver Functions for Pharmacokinetics and Safety Evaluation of Pharmaceuticals.Curr Pharm Biotechnol. 2020;21(9):773-779. doi: 10.2174/1389201021666200131123524. Curr Pharm Biotechnol. 2020. PMID: 32003687 Review.
Cited by
-
Robust expansion and functional maturation of human hepatoblasts by chemical strategy.Stem Cell Res Ther. 2021 Feb 25;12(1):151. doi: 10.1186/s13287-021-02233-9. Stem Cell Res Ther. 2021. PMID: 33632328 Free PMC article.
-
Chemically-Defined, Xeno-Free, Scalable Production of hPSC-Derived Definitive Endoderm Aggregates with Multi-Lineage Differentiation Potential.Cells. 2019 Dec 4;8(12):1571. doi: 10.3390/cells8121571. Cells. 2019. PMID: 31817235 Free PMC article.
-
A Small-Molecule Cocktails-Based Strategy in Culture of Mesenchymal Stem Cells.Front Bioeng Biotechnol. 2022 Mar 14;10:819148. doi: 10.3389/fbioe.2022.819148. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35360405 Free PMC article.
-
Current Perspective: 3D Spheroid Models Utilizing Human-Based Cells for Investigating Metabolism-Dependent Drug-Induced Liver Injury.Front Med Technol. 2020 Nov 30;2:611913. doi: 10.3389/fmedt.2020.611913. eCollection 2020. Front Med Technol. 2020. PMID: 35047893 Free PMC article. Review.
-
Human Pluripotent Stem Cell-Derived Hepatocyte-Like Cells and Organoids for Liver Disease and Therapy.Int J Mol Sci. 2021 Sep 28;22(19):10471. doi: 10.3390/ijms221910471. Int J Mol Sci. 2021. PMID: 34638810 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

