Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells
- PMID: 29523190
- PMCID: PMC5845143
- DOI: 10.1186/s13287-018-0797-1
Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells
Abstract
Background: Intervertebral disc (IVD) degeneration is characterized by an early decrease in cellularity of the nucleus pulposus (NP) region, and associated extracellular matrix changes, reduced hydration, and progressive degeneration. Cell-based IVD therapy has emerged as an area of great interest, with studies reporting regenerative potential for many cell sources, including autologous or allogeneic chondrocytes, primary IVD cells, and stem cells. Few approaches, however, have clear strategies to promote the NP phenotype, in part due to a limited knowledge of the defined markers and differentiation protocols for this lineage. Here, we developed a new protocol for the efficient differentiation of human induced pluripotent stem cells (hiPSCs) into NP-like cells in vitro. This differentiation strategy derives from our knowledge of the embryonic notochordal lineage of NP cells as well as strategies used to support healthy NP cell phenotypes for primary cells in vitro.
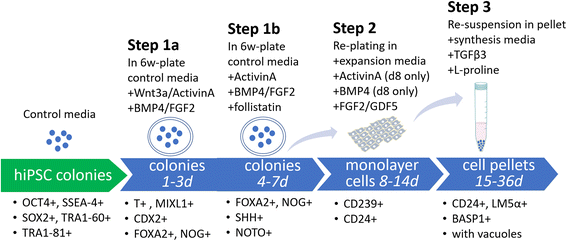
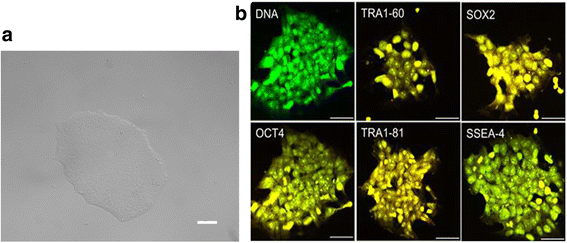
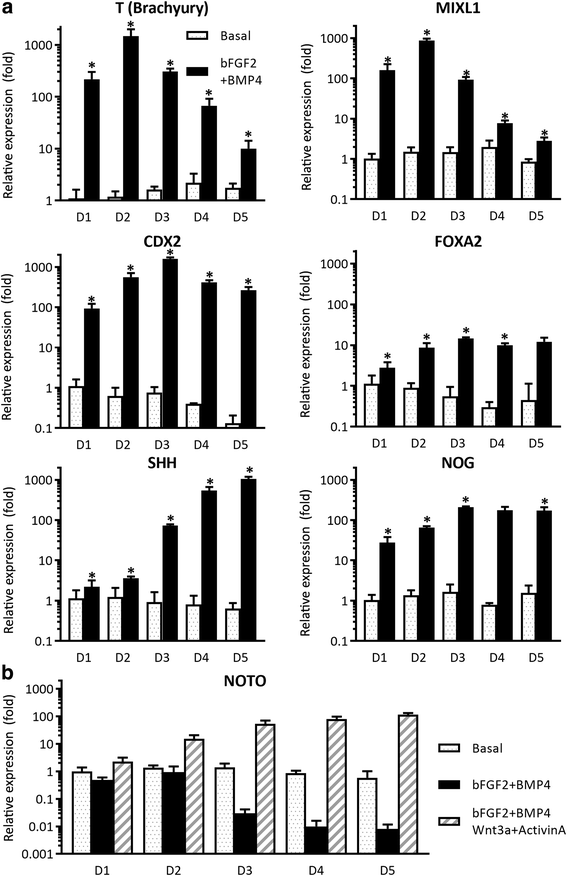
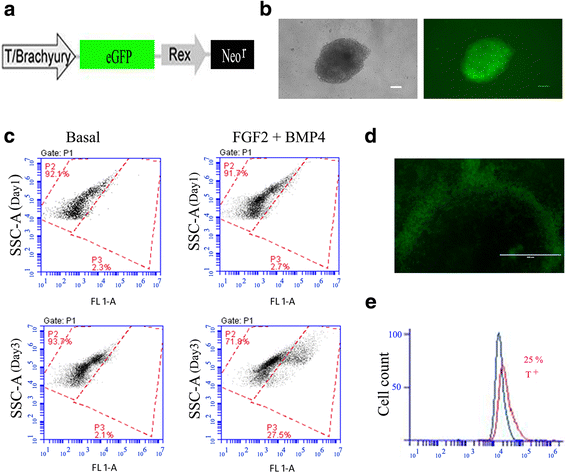
Methods: An NP-genic phenotype of hiPSCs was promoted in undifferentiated hiPSCs using a stepwise, directed differentiation toward mesodermal, and subsequently notochordal, lineages via chemically defined medium and growth factor supplementation. Fluorescent cell imaging was used to test for pluripotency markers in undifferentiated cells. RT-PCR was used to test for potential cell lineages at the early stage of differentiation. Cells were checked for NP differentiation using immunohistochemistry and histological staining at the end of differentiation. To enrich notochordal progenitor cells, hiPSCs were transduced using lentivirus containing reporter constructs for transcription factor brachyury (T) promoter and green fluorescent protein (GFP) fluorescence, and then sorted on T expression based on GFP intensity by flow cytometry.
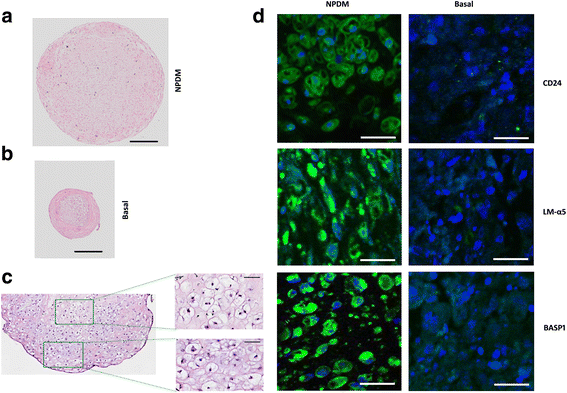
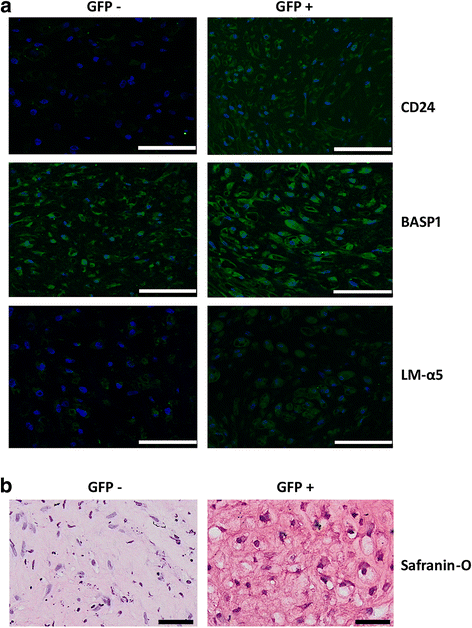
Results: Periods of pellet culture following initial induction were shown to promote the vacuolated NP cell morphology and NP surface marker expression, including CD24, LMα5, and Basp1. Enrichment of brachyury (T) positive cells using fluorescence-activated cell sorting was shown to further enhance the differentiation efficiency of NP-like cells.
Conclusions: The ability to efficiently differentiate human iPSCs toward NP-like cells may provide insights into the processes of NP cell differentiation and provide a cell source for the development of new therapies for IVD diseases.
Keywords: Cartilage; Chondrocyte; Degenerative disc disease; Directed differentiation; Human induced pluripotent stem cells; Intervertebral disc; Nucleus pulposus.
Conflict of interest statement
Ethics approval and consent to participate
The use of this cell line was approved as not human subjects research in accordance with institutional policies at Duke University and at Washington University in St. Louis.
Consent for publication
Not applicable.
Competing interests
VPW and FG are paid employees of Cytex Therapeutics. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration.J Biomed Mater Res A. 2015 Mar;103(3):1053-9. doi: 10.1002/jbm.a.35243. Epub 2014 Jun 17. J Biomed Mater Res A. 2015. PMID: 24889905
-
The generation and functional characterization of induced pluripotent stem cells from human intervertebral disc nucleus pulposus cells.Oncotarget. 2017 Jun 27;8(26):42700-42711. doi: 10.18632/oncotarget.17446. Oncotarget. 2017. PMID: 28498811 Free PMC article.
-
Differentiation of Pluripotent Stem Cells into Nucleus Pulposus Progenitor Cells for Intervertebral Disc Regeneration.Curr Stem Cell Res Ther. 2019;14(1):57-64. doi: 10.2174/1574888X13666180918095121. Curr Stem Cell Res Ther. 2019. PMID: 30227822 Review.
-
Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model.Theranostics. 2019 Oct 12;9(25):7506-7524. doi: 10.7150/thno.34898. eCollection 2019. Theranostics. 2019. PMID: 31695783 Free PMC article.
-
The nucleus pulposus microenvironment in the intervertebral disc: the fountain of youth?Eur Cell Mater. 2021 Jun 15;41:707-738. doi: 10.22203/eCM.v041a46. Eur Cell Mater. 2021. PMID: 34128534 Review.
Cited by
-
Biomaterials and Cell-Based Regenerative Therapies for Intervertebral Disc Degeneration with a Focus on Biological and Biomechanical Functional Repair: Targeting Treatments for Disc Herniation.Cells. 2022 Feb 9;11(4):602. doi: 10.3390/cells11040602. Cells. 2022. PMID: 35203253 Free PMC article. Review.
-
Development, Pathogenesis, and Regeneration of the Intervertebral Disc: Current and Future Insights Spanning Traditional to Omics Methods.Front Cell Dev Biol. 2022 Mar 11;10:841831. doi: 10.3389/fcell.2022.841831. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35359439 Free PMC article. Review.
-
Development of the axial skeleton and intervertebral disc.Curr Top Dev Biol. 2019;133:49-90. doi: 10.1016/bs.ctdb.2018.11.018. Epub 2019 Jan 3. Curr Top Dev Biol. 2019. PMID: 30902259 Free PMC article. Review.
-
Neuroprotective Strategies for Retinal Ganglion Cell Degeneration: Current Status and Challenges Ahead.Int J Mol Sci. 2020 Mar 25;21(7):2262. doi: 10.3390/ijms21072262. Int J Mol Sci. 2020. PMID: 32218163 Free PMC article. Review.
-
Versatility of Induced Pluripotent Stem Cells (iPSCs) for Improving the Knowledge on Musculoskeletal Diseases.Int J Mol Sci. 2020 Aug 25;21(17):6124. doi: 10.3390/ijms21176124. Int J Mol Sci. 2020. PMID: 32854405 Free PMC article. Review.
References
-
- Katz J. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;S2:21–4. - PubMed
-
- Lazebnik M, Singh M, Glatt P, Friis LA, Berkland CJ, Detamore MS. Biomimetic method for combining the nucleus pulposus and annulus fibrosus for intervertebral disc tissue engineering. J Tissue Eng Regen Med. 2011;5:e179–87. 10.1002/term.412 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

