Radial shockwave treatment promotes human mesenchymal stem cell self-renewal and enhances cartilage healing
- PMID: 29523197
- PMCID: PMC5845163
- DOI: 10.1186/s13287-018-0805-5
Radial shockwave treatment promotes human mesenchymal stem cell self-renewal and enhances cartilage healing
Abstract
Background: Shockwaves and mesenchymal stem cells (MSCs) have been widely accepted as useful tools for many orthopedic applications. However, the modulatory effects of shockwaves on MSCs remain controversial. In this study, we explored the influence of radial shockwaves on human bone marrow MSCs using a floating model in vitro and evaluated the healing effects of these cells on cartilage defects in vivo using a rabbit model.
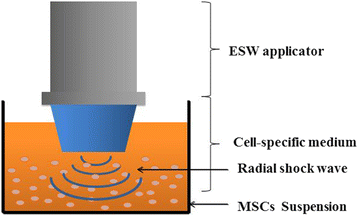
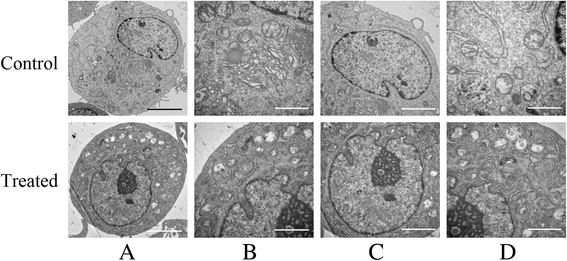
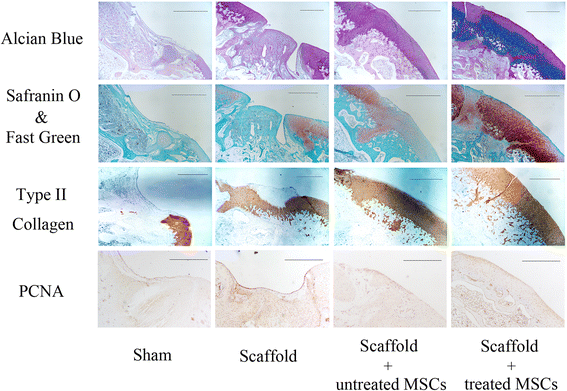
Methods: MSCs were cultured in vitro, harvested, resuspended, and treated with various doses of radial shockwaves in a floating system. Cell proliferation was evaluated by growth kinetics and Cell Counting Kit-8 (CCK-8) assay. In addition, the cell cycle and apoptotic activity were analyzed by fluorescence activated cell sorting. To explore the "stemness" of MSCs, cell colony-forming tests and multidifferentiation assays were performed. We also examined the MSC subcellular structure using transmission electron microscopy and examined the healing effects of these cells on cartilage defects by pathological analyses.
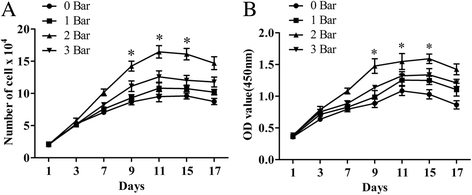
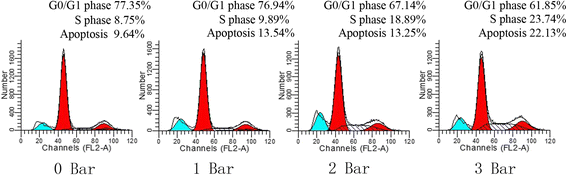
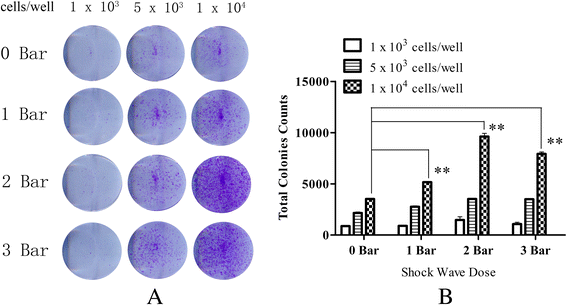
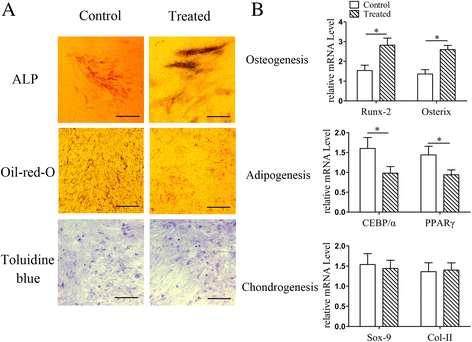
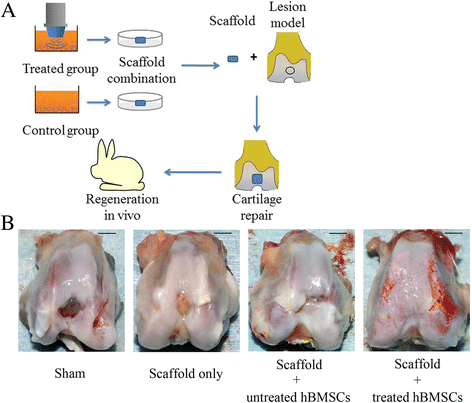
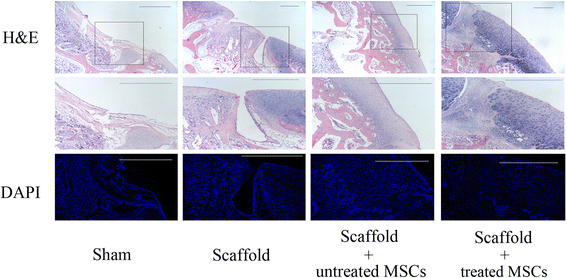
Results: The results of growth kinetics and CCK-8 assays showed that radial shockwave treatment significantly promoted MSC proliferation. Enhanced cell growth was also reflected by an increase in the numbers of cells in the S phase and a decrease in the numbers of cells arrested in the G0/G1 phase in shockwave-treated MSCs. Unexpectedly, shockwaves caused a slight increase in MSC apoptosis rates. Furthermore, radial shockwaves promoted self-replicating activity of MSCs. Transmission electron microscopy revealed that MSCs were metabolically activated by shockwave treatment. In addition, radial shockwaves favored MSC osteogenic differentiation but inhibited adipogenic activity. Most importantly, MSCs pretreated by radial shockwaves exhibited an enhanced healing effect on cartilage defects in vivo. Compared with control groups, shockwave-treated MSCs combined with bio-scaffolds significantly improved histological scores of injured rabbit knees.
Conclusions: In the present study, we found that radial shockwaves significantly promoted the proliferation and self-renewal of MSCs in vitro and safely accelerated the cartilage repair process in vivo, indicating favorable clinical outcomes.
Keywords: Cartilage repair; Mesenchymal stem cell; Radial shockwave.
Conflict of interest statement
Ethics approval
Informed consent was obtained from all patients for research purposes, and experiments were approved by the Ethics Review Committee of the PLA General Hospital. All animal experimental protocols were in compliance with the Animal Welfare Act and were approved by the Animal Care and Use Committee of the Laboratory Animal Research Center at the PLA General Hospital (Reference number: 2015-X11-10).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Bhattacharjee M, Coburn J, Centola M, Murab S, Barbero A, Kaplan DL, Martin I, Ghosh S. Tissue engineering strategies to study cartilage development, degeneration and regeneration. Adv Drug Deliv Rev. 2015;84:107–22. 10.1016/j.addr.2014.08.010. - PubMed
-
- de Windt TS, Hendriks JA, Zhao X, Vonk LA, Creemers LB, Dhert WJ, Randolph MA, Saris DB. Concise review: Unraveling stem cell cocultures in regenerative medicine: which cell interactions steer cartilage regeneration and how? Stem Cells Transl Med. 2014;3:723–733. doi: 10.5966/sctm.2013-0207. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

