Pathologic and biochemical characterization of PrPSc from elk with PRNP polymorphisms at codon 132 after experimental infection with the chronic wasting disease agent
- PMID: 29523205
- PMCID: PMC5845354
- DOI: 10.1186/s12917-018-1400-9
Pathologic and biochemical characterization of PrPSc from elk with PRNP polymorphisms at codon 132 after experimental infection with the chronic wasting disease agent
Abstract
Background: The Rocky Mountain elk (Cervus elaphus nelsoni) prion protein gene (PRNP) is polymorphic at codon 132, with leucine (L132) and methionine (M132) allelic variants present in the population. In elk experimentally inoculated with the chronic wasting disease (CWD) agent, different incubation periods are associated with PRNP genotype: LL132 elk survive the longest, LM132 elk are intermediate, and MM132 elk the shortest. The purpose of this study was to investigate potential mechanisms underlying variations in incubation period in elk of different prion protein genotypes. Elk calves of three PRNP genotypes (n = 2 MM132, n = 2 LM132, n = 4 LL132) were orally inoculated with brain homogenate from elk clinically affected with CWD.
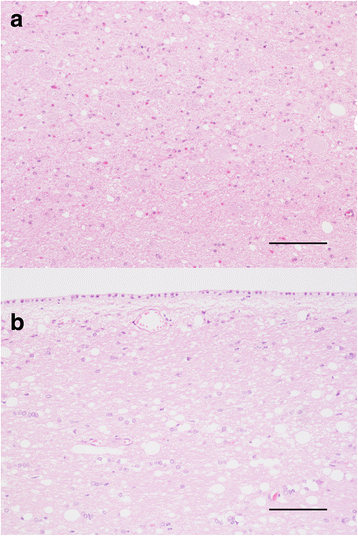
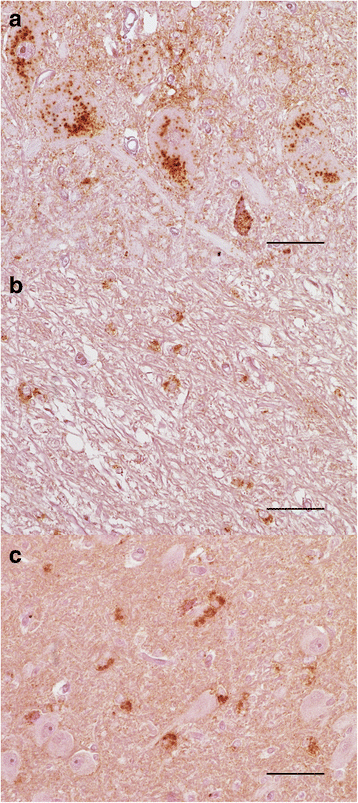
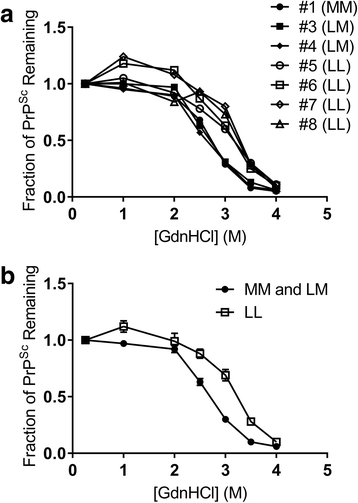
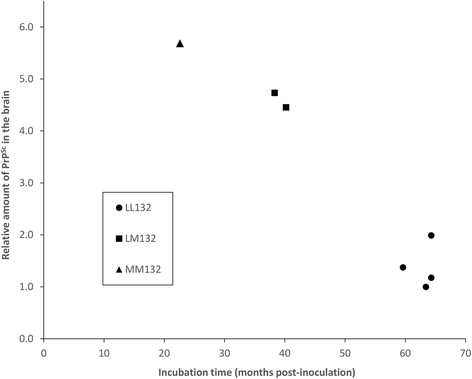
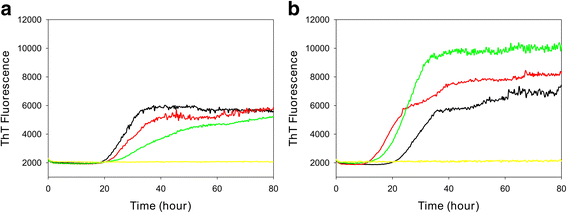
Results: Elk with longer incubation periods accumulated relatively less PrPSc in the brain than elk with shorter incubation periods. PrPSc accumulation in LM132 and MM132 elk was primarily neuropil-associated while glial-associated immunoreactivity was prominent in LL132 elk. The fibril stability of PrPSc from MM132 and LM132 elk were similar to each other and less stable than that from LL132 elk. Real-time quaking induced conversion assays (RT-QuIC) revealed differences in the ability of PrPSc seed from elk of different genotypes to convert recombinant 132 M or 132 L substrate.
Conclusions: This study provides further evidence of the importance of PRNP genotype in the pathogenesis of CWD of elk. The longer incubation periods observed in LL132 elk are associated with PrPSc that is more stable and relatively less abundant at the time of clinical disease. The biochemical properties of PrPSc from MM132 and LM132 elk are similar to each other and different to PrPSc from LL132 elk. The shorter incubation periods in MM132 compared to LM132 elk may be the result of genotype-dependent differences in the efficiency of propagation of PrPSc moieties present in the inoculum. A better understanding of the mechanisms by which the polymorphisms at codon 132 in elk PRNP influence disease pathogenesis will help to improve control of CWD in captive and free-ranging elk populations.
Keywords: Chronic wasting disease; Conformational stability; Elk; Prion protein; RT-QuIC.
Conflict of interest statement
Ethics approval and consent to participate
This experiment was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Washington, DC) and the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, Champaign, IL). The Institutional Animal Care and Use Committee at the National Animal Disease Center reviewed and approved the animal use protocol (protocol number: 3833).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Quantifying the Molecular Properties of the Elk Chronic Wasting Disease Agent with Mass Spectrometry.Pathogens. 2024 Nov 16;13(11):1008. doi: 10.3390/pathogens13111008. Pathogens. 2024. PMID: 39599561 Free PMC article.
-
Novel Strain of the Chronic Wasting Disease Agent Isolated From Experimentally Inoculated Elk With LL132 Prion Protein.Sci Rep. 2020 Feb 21;10(1):3148. doi: 10.1038/s41598-020-59819-1. Sci Rep. 2020. PMID: 32081886 Free PMC article.
-
Detection of the abnormal isoform of the prion protein associated with chronic wasting disease in the optic pathways of the brain and retina of Rocky Mountain elk (Cervus elaphus nelsoni).Vet Pathol. 2010 May;47(3):536-46. doi: 10.1177/0300985810363702. Epub 2010 Apr 9. Vet Pathol. 2010. PMID: 20382822
-
Cervid Prion Protein Polymorphisms: Role in Chronic Wasting Disease Pathogenesis.Int J Mol Sci. 2021 Feb 25;22(5):2271. doi: 10.3390/ijms22052271. Int J Mol Sci. 2021. PMID: 33668798 Free PMC article. Review.
-
Chronic wasting disease.Biochim Biophys Acta. 2007 Jun;1772(6):610-8. doi: 10.1016/j.bbadis.2006.10.010. Epub 2006 Oct 18. Biochim Biophys Acta. 2007. PMID: 17223321 Free PMC article. Review.
Cited by
-
Association Study of the M132L Single Nucleotide Polymorphism With Susceptibility to Chronic Wasting Disease in Korean Elk: A Meta-Analysis.Front Vet Sci. 2022 Jan 14;8:804325. doi: 10.3389/fvets.2021.804325. eCollection 2021. Front Vet Sci. 2022. PMID: 35097050 Free PMC article.
-
Quantifying the Molecular Properties of the Elk Chronic Wasting Disease Agent with Mass Spectrometry.Pathogens. 2024 Nov 16;13(11):1008. doi: 10.3390/pathogens13111008. Pathogens. 2024. PMID: 39599561 Free PMC article.
-
Overview of North American Isolates of Chronic Wasting Disease Used for Strain Research.Pathogens. 2025 Mar 4;14(3):250. doi: 10.3390/pathogens14030250. Pathogens. 2025. PMID: 40137736 Free PMC article. Review.
-
Identification of circulating microRNA signatures as potential biomarkers in the serum of elk infected with chronic wasting disease.Sci Rep. 2019 Dec 23;9(1):19705. doi: 10.1038/s41598-019-56249-6. Sci Rep. 2019. PMID: 31873177 Free PMC article.
-
Novel Strain of the Chronic Wasting Disease Agent Isolated From Experimentally Inoculated Elk With LL132 Prion Protein.Sci Rep. 2020 Feb 21;10(1):3148. doi: 10.1038/s41598-020-59819-1. Sci Rep. 2020. PMID: 32081886 Free PMC article.
References
-
- Dassanayake RP, Orru CD, Hughson AG, Caughey B, Graca T, Zhuang D, Madsen-Bouterse SA, Knowles DP, Schneider DA. Sensitive and specific detection of classical scrapie prions in the brains of goats by real-time quaking-induced conversion. J Gen Virol. 2016;97(3):803–812. doi: 10.1099/jgv.0.000367. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

