Effectiveness and cost-effectiveness of humanistic counselling in schools for young people with emotional distress (ETHOS): study protocol for a randomised controlled trial
- PMID: 29523206
- PMCID: PMC5845203
- DOI: 10.1186/s13063-018-2538-2
Effectiveness and cost-effectiveness of humanistic counselling in schools for young people with emotional distress (ETHOS): study protocol for a randomised controlled trial
Abstract
Background: One in ten children in Britain have been identified as experiencing a diagnosable mental health disorder. School-based humanistic counselling (SBHC) may help young people identify, address, and overcome psychological distress. Data from four pilot trials suggest that SBHC may be clinically effective. However, a fully powered randomised controlled trial (RCT) is needed to provide a robust test of its effectiveness, to assess its cost-effectiveness, and to determine the process of change.
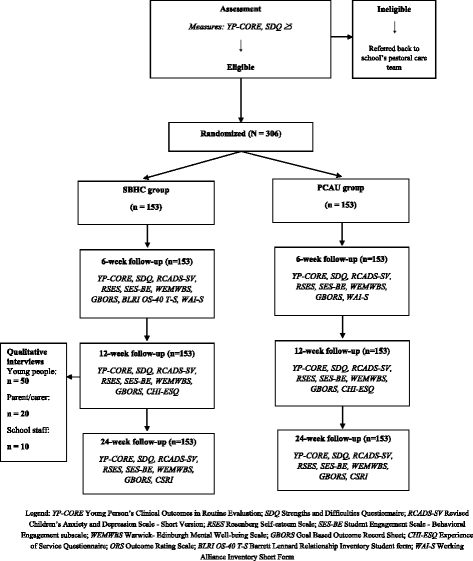
Methods/design: The Effectiveness and Cost-effectiveness Trial of Humanistic Counselling in Schools (ETHOS) is a two-arm, parallel-group RCT comparing the clinical and cost-effectiveness of SBHC with Pastoral Care as Usual (PCAU) in school settings. Eligibility criteria for young people include being between 13 and 16 years of age and experiencing moderate to severe levels of emotional distress. Participants are randomised to receive either SBHC or PCAU. SBHC is delivered in up to 10 weekly, individual sessions in their school with a qualified, experienced counsellor who has also received training using a clinical practice manual. Adherence to the SBHC model is assessed by a sub-team of auditors and in clinical supervision. PCAU consists of the schools' pre-existing systems for supporting the emotional health and well-being of students. The primary outcomes are psychological distress measured using the Young Person's Clinical Outcomes in Routine Evaluation (YP-CORE) and costs evaluated using the Client Service Receipt Inventory (CSRI). Secondary outcomes include psychological difficulties, levels of depression, anxiety and self-esteem, well-being, school engagement, educational outcomes and achievement of personal goals. Qualitative interviews with participants, parents and school staff will look to identify the mechanisms of change in SBHC. Researchers administering the measures are blind to allocation. The trial requires n = 306 participants (n = 153 in each group), with 90% power to detect a standardised mean difference (SMD) of 0.5. An intention-to-treat analysis will be undertaken.
Discussion: This RCT is powered to detect clinically meaningful differences, and will make a major contribution to the evidence base for mental health provision for adolescents. It will have implications for all stakeholders, including policy-makers, statutory advisory bodies for child welfare, head teachers, children and young people practitioners, child welfare and parenting organisations, and young people.
Trial registration: Controlled Trials International Standard Randomised Controlled Trial Number (ISRCTN) Registry, ID: ISRCTN10460622 . Registered on 11 May 2016.
Keywords: Cost-effectiveness; Psychological distress; Randomised controlled trial; School-based humanistic counselling; Young people.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval for the trial has been obtained by the University of Roehampton, Department of Psychology, Ethics Committee (PSYC 16/ 227, 31 August 2016). Informed consent to participate in the study is obtained from all young people and their parents/carers before they take part in the study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Green H, McGinnity A, Meltzer H, Ford T, Goodman R. Mental health of children and young people in Great Britain. Office for National Statistics. Hampshire, UK: Palgrave MacMillan; 2004.
-
- The Prince’s Trust. The Prince’s Trust Youth Index. 2012. https://www.princes-trust.org.uk/. Accessed 9 Sep 2014.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

