Pharmacokinetics of amlodipine besylate at delivery and during lactation
- PMID: 29523279
- PMCID: PMC5846630
- DOI: 10.1016/j.preghy.2018.01.002
Pharmacokinetics of amlodipine besylate at delivery and during lactation
Abstract
Background: Amlodipine is rarely used in the treatment of pregnant hypertensive women due to limited pharmacokinetic data during pregnancy and the postpartum period.
Objective: To evaluate the pharmacokinetics of amlodipine besylate in the peri-partum period including quantities of placental passage, breast milk excretion and infant exposure.
Study design: This was a prospective study of pregnant women who were prescribed 5 mg of amlodipine daily for treatment of chronic hypertension and delivered at term. Cord and maternal blood samples were collected at delivery. On postpartum day 2, six paired maternal plasma and breast milk samples were obtained at 4, 6, 8, 12, 15 and 24 h following amlodipine dosing. Infant plasma samples were collected 24-48 h after delivery. All samples were analyzed for amlodipine concentration. A one compartment, first-order model was used to calculate pharmacokinetic estimates for maternal plasma.
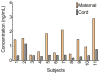
Results: Of the 16 patients enrolled in the study, 11 had cord blood and maternal serum collected at delivery, of which only 6 produced sufficient breast milk for sampling. Amlodipine was detected in infant cord blood plasma with a mean concentration of 0.49 ± 0.29 ng/mL compared to mean maternal serum level of 1.27 ± 0.84 ng/mL. Amlodipine concentrations in both in breast milk and infant plasma were undetectable at the lower limit of assay detection (<0.1 ng/mL). In the immediate postpartum period, the amlodipine elimination half-life was 13.7 ± 4.9 h, the area under the curve was 53.4 ± 19.8 ng*h/mL and the peak concentration was 2.0 ± 1.0 ng/mL.
Conclusions: Amlodipine does cross the placenta in measurable quantities, but is not detected in breast milk or infant plasma at 24-48 h of life indicating that it is likely safe to use during the peripartum period.
Keywords: Amlodipine; Breast milk; Chronic hypertension; Pharmacokinetics.
Copyright © 2018 International Society for the Study of Hypertension in Pregnancy. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest statement: The authors report no conflicts of interest
Figures

References
-
- Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy: Executive Summary. Obstet Gynecol. 2013;122:1122–1131. - PubMed
-
- National Health and Nutrition Examination Survey (NHANES) Centers for Disease Control and Prevention. 2011 Accessed May 21, 2014.
-
- Norvasc Product Monograph. Pfizer Canada, Inc; Kirkland, Quebec: Available at http://www.pfizer.ca/sites/g/files/g10017036/f/201505/Norvasc_PM_E_17771.... Accessed May 24, 2014.
-
- Burnett A. Norvasc Drug Monograph. University of New Mexico College of Pharmacy; Albuquerque, New Mexico: Accessed May 23, 2014.
-
- Consumer Reports Health Best Buy Drugs, “Using Calcium Channel Blockers to treat high blood pressure and heart disease: comparing effectiveness, safety and price”. Best Buy Drugs (Consumer Reports) accessed May 11, 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

