Mitochondria-Endoplasmic Reticulum Contact Sites Function as Immunometabolic Hubs that Orchestrate the Rapid Recall Response of Memory CD8+ T Cells
- PMID: 29523440
- PMCID: PMC6049611
- DOI: 10.1016/j.immuni.2018.02.012
Mitochondria-Endoplasmic Reticulum Contact Sites Function as Immunometabolic Hubs that Orchestrate the Rapid Recall Response of Memory CD8+ T Cells
Abstract
Glycolysis is linked to the rapid response of memory CD8+ T cells, but the molecular and subcellular structural elements enabling enhanced glucose metabolism in nascent activated memory CD8+ T cells are unknown. We found that rapid activation of protein kinase B (PKB or AKT) by mammalian target of rapamycin complex 2 (mTORC2) led to inhibition of glycogen synthase kinase 3β (GSK3β) at mitochondria-endoplasmic reticulum (ER) junctions. This enabled recruitment of hexokinase I (HK-I) to the voltage-dependent anion channel (VDAC) on mitochondria. Binding of HK-I to VDAC promoted respiration by facilitating metabolite flux into mitochondria. Glucose tracing pinpointed pyruvate oxidation in mitochondria, which was the metabolic requirement for rapid generation of interferon-γ (IFN-γ) in memory T cells. Subcellular organization of mTORC2-AKT-GSK3β at mitochondria-ER contact sites, promoting HK-I recruitment to VDAC, thus underpins the metabolic reprogramming needed for memory CD8+ T cells to rapidly acquire effector function.
Keywords: Akt; GSK3-beta; IFN-gamma; VDAC; endoplasmic reticulum; glycolysis; hexokinase; mTOR; memory CD8(+) T cells; mitochondria.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
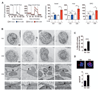



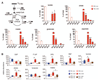


Comment in
-
Mitochondria-ER Pas de Deux Controls Memory T Cell Function.Immunity. 2018 Mar 20;48(3):479-481. doi: 10.1016/j.immuni.2018.03.002. Immunity. 2018. PMID: 29562198
References
-
- Arzoine L, Zilberberg N, Ben-Romano R, Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with hexokinase to prevent its anti-apoptotic activity. The Journal of biological chemistry. 2009;284:3946–3955. - PubMed
-
- Bagchi S, Fredriksson R, Wallen-Mackenzie A. In Situ Proximity Ligation Assay (PLA) Methods Mol Biol. 2015;1318:149–159. - PubMed
-
- Balmer ML, Hess C. Feeling Worn Out? PGC1alpha to the Rescue for Dysfunctional Mitochondria in T Cell Exhaustion. Immunity. 2016;45:233–235. - PubMed
-
- Balmer ML, Ma EH, Bantug GR, Grahlert J, Pfister S, Glatter T, Jauch A, Dimeloe S, Slack E, Dehio P, et al. Memory CD8(+) T Cells Require Increased Concentrations of Acetate Induced by Stress for Optimal Function. Immunity. 2016;44:1312–1324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

